Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/22/02. The contractual start date was in November 2018. The draft report began editorial review in January 2021 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Norman et al. This work was produced by Norman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Norman et al.
Chapter 1 Introduction
Scientific background
Methods of birth in preterm birth
Preterm birth (PTB) (prior to 37 weeks’ gestation) affects 7% of UK livebirths and is the single largest cause of neonatal mortality and morbidity. Survival to 1 year of life and rates of disability are inversely proportional to length of gestation (i.e. babies born at lower gestational ages do worse than those born at higher gestational ages). Importantly, although survival rates have increased with time, rates of disability have remained unchanged. 1,2
Despite the relatively common nature of PTB, there is significant uncertainty about which mode of birth (MoB) [vaginal or caesarean section (CS)] is best. Current guidance advises clinicians to discuss the risks and benefits of vaginal and caesarean birth with women thought to be in preterm labour and to highlight the potential risks associated with CSs.
Despite this advice, the evidence base on risks and benefits is limited largely to observational studies. There is uncertainty as to whether or not a randomised trial is possible, in part because of established practice.
The research described in this monograph was in response to a Health Technology Assessment commissioned call (17/22 ‘Mode of delivery for preterm infants’) to:
. . . establish the scenarios in which there is equipoise in how best to deliver a preterm baby and to define the most important outstanding question(s) for clinicians and parents in this area that could be addressed by a future trial. If outstanding questions are identified in this first phase, then researchers are asked to conduct qualitative work with clinicians and potential participants to determine the acceptability of randomisation in order to inform the feasibility of future research.
Uncertainty about the best-planned mode of birth for women in spontaneous preterm labour
The majority of PTBs follow the premature initiation of spontaneous labour. There is clinical uncertainty about the optimal MoB in this scenario. A minority of women require CS (e.g. those with fulminating pre-eclampsia), and these women are not the focus of this study. For the remainder of women, there is significant clinical uncertainty, and some clinicians believe that birth by CS is best because of the hypothesised reduction in birth trauma and intrapartum hypoxia. Others believe that vaginal birth confers advantages for the baby (e.g. reducing respiratory morbidity), the mother (e.g. avoiding operative complications) and the NHS (e.g. costs). There are similar uncertainties about the best mode of planned PTB. Addressing these clinical uncertainties could significantly improve the health of the public and patients. Rates of intrapartum stillbirth and neonatal and long-term mortality and morbidity are higher in the 60,000 preterm babies born in the UK each year than with term babies.
In addition to these clinical uncertainties, there is very little evidence on the best MoB. To the best of our knowledge, there is only one systematic review3 of randomised trials on this topic. In this systematic review,3 only four studies (involving only 116 women) were considered to be sufficiently robust to be able contribute data to the analysis, and the most recent study was conducted 25 year ago. There were very few data of relevance to the two main (primary) outcomes for the baby considered in the review [i.e. birth injury to infant and birth asphyxia (as defined by the triallists)]. For the mother, there were very few data on the primary outcome of admission to intensive care/major maternal post-partum complications; however, women in the vaginal birth group had lower rates of puerperal pyrexia [relative risk (RR) 2.98, 95% confidence interval (CI) 1.18 to 7.53; three trials, n = 89 women] and other maternal infection (RR 2.63, 95% CI 1.02 to 6.78; three trials, n = 103 women). The authors concluded that ‘there is not enough evidence to evaluate the use of a policy of planned immediate caesarean birth for preterm babies. Further studies are needed in this area’. 3
The Cochrane systematic review was updated by the Guideline Development Group for the National Institute for Health and Care Excellence (NICE) Preterm Labour and Birth guideline (2015),4 which found no new randomised trials. A further update of this search was conducted in November 2017 prior to submission of the full grant application for this study using the medical subject headings premature birth AND delivery; obstetric AND randomised trial; premature birth AND caesarean delivery AND randomised trial; premature birth AND labor AND obstetric AND randomised trial, with publication date of January 2011–November 2017 (see Appendix 1). Again, we found no new randomised trials to address the question of the best MoB for women in preterm labour or undergoing planned PTB. A further scoping search was undertaken in preparation for the Delphi exercise on 18 May 2019 and, again, no new randomised trials were found. Both of our own searches identified some observational studies. An initial review of these observational studies shows the extent of the controversy, with evidence both of worse outcomes5,6 and of better outcomes7,8 in babies delivered by CS than in babies delivered vaginally, and also evidence of no difference. 9 It is plausible that planned birth by CS could reduce the frequency of either death or disability in preterm babies compared with the control standard of care of vaginal birth. Indeed, our recent retrospective study of 1575 UK babies born between 23 and 27 weeks’ gestation found that, after adjusting for confounders, babies born vaginally had a higher odds (OR 1.61, 95% CI 1.01 to 2.58) of intraventricular haemorrhage (IVH). 8 Another study has shown that neonatal mortality is lower in babies born by CS than in babies born vaginally. 10 Conversely, CS is associated with higher NHS costs and greater complications for the mother,11 and there is conflicting evidence of benefit for preterm babies. 3,5,6
Importantly, there is uncertainty about the subgroups of women (e.g. those with cephalic presentation only, those at a particular gestational age and those without any signs of intrauterine growth restriction) for whom there is equipoise about the appropriate MoB. A brief description of these subgroups of women follows. First, there may be one or more babies. The Cochrane review12 and the NICE guideline4 focused on singleton pregnancy (i.e. just one baby), and we intended to include discussion about multiple pregnancy in our research in order not to narrow the question too quickly. Second, the baby (or babies) may be presenting by the breech or cephalic. (It is assumed that babies with a non-longitudinal lie would require birth by CS.) Three of the four studies in the Cochrane review12 focused on babies with breech presentation. There is arguably less controversy about the optimal MoB in women delivering preterm with breech presentation, as randomised trials of term babies have provided evidence of the advantages of CS for these babies. 13 Indeed, the NICE guideline Preterm Labour and Birth4 acknowledges this and suggests that, for women in preterm labour with breech presentation, CS should be ‘considered’. 4 Last, it is plausible that the risks and benefits of CS and vaginal birth may be different when comparing women who are in preterm labour with women undergoing planned PTB. Both subsets of women are included in the commissioning brief and we have addressed both in our research described here.
Despite the lack of evidence and uncertainty in the national guidance,11 it is not clear whether clinicians and pregnant women are in equipoise about the best MoB. Acknowledging that published evidence is only one source of information that clinicians and women use for clinical decision-making, the purpose of this study was to determine ‘[i]n which groups of women and babies in preterm labour is there clinical uncertainty about planned mode of birth’.
Uncertainty about whether or not a trial to compare planned modes of birth is feasible
In addition to the uncertainty about the best-planned MoB, there is uncertainty about whether or not a trial to compare planned modes of birth is feasible. Clinicians may not wish to randomise women to CS or vaginal birth because they have firm beliefs about the optimal MoB (despite the lack of published evidence). Likewise, pregnant women may not wish randomisation for a variety of reasons, including the experience of friends or family. The uncertainty about whether or not women and clinicians would accept randomisation has been given prominence by the Cochrane review, which noted that ‘[f]urther studies are needed in this area [of best method of delivery], but recruitment is proving difficult’. 3 The comment on difficult recruitment arose because all four of the randomised trials, which provided data for the Cochrane review, closed without having reached their sample size. However, three14–16 out of four of these studies focused on women with breech presentation for whom there is less clinical uncertainty (as described above). The most recent of these studies (of breech presentation) was published over 20 years ago. 14 In addition, the rationale for early termination of the study of singleton babies with cephalic presentation was because of an ‘unacceptably high proportion (63%) of babies with birthweight > 1500 g’ and not because of poor recruitment. This fourth trial was published over 30 years ago. 17 Therefore, there is little evidence to determine whether or not recruitment to a randomised trial of MoB is feasible, and much of the available evidence is from women delivering over 25 years ago.
This project aims to determine which groups of women in preterm labour or with planned PTB would be willing to randomise to a potential future trial. Randomised trials have been performed to address optimal MoB for women with breech presentation13 and women with twin pregnancy,18 and these trials have (arguably) reduced uncertainty. However, there are few randomised trials to compare planned CS with vaginal birth for women with a singleton pregnancy and cephalic presentation at term. 19 Importantly, in a previous study of women with a previous CS at full-term gestation, comparing elective repeat CS with vaginal birth, the majority of women were allocated by patient preference rather than randomisation, implying reluctance to randomise or be randomised. 20
Rationale for research
Aims
This project, which we called the CASSAVA project, was funded by the National Institute for Health Research as part of the Health Technology Assessment programme. The overall aim of the project was to find out the groups of women and babies in preterm labour for whom there is clinical uncertainty about the optimal planned mode of birth and whether or not women and clinical staff would be willing to participate in a future randomised trial to address this question. We aimed to determine which of the four statements below is most accurate and to define any uncertainties:
-
There are no uncertainties about the best-planned MoB for any groups of women or babies presenting in preterm labour.
-
There are uncertainties about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, and in the willingness of clinicians to recruit to, and of women to participate in, a randomised trial to address these uncertainties.
-
There are uncertainties about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, and in the willingness of clinicians to recruit to, and of women to participate in, a randomised trial to address some, but not all, of these uncertainties.
-
There is uncertainty about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, but women and/or clinical staff are not willing to participate in a randomised trial to address any of these uncertainties.
Objectives
To achieve our overall aim, our stated objectives were as follows:
-
To perform two surveys: one survey with health-care professionals to establish current practice and opinion in key clinical scenarios of women presenting in preterm labour (e.g. cephalic/breech, previous CS or not, growth restriction present or not, gestational age of 23–24, 24–28 or 28–36 weeks) (see Chapter 3) and a second survey with parents to establish current opinion about the best MoB (see Chapter 4).
-
To convene an interactive working group of clinicians to determine what kind of trials still need to be carried out and what groups of women should be included in these trials. Formal consensus methodology was used to resolve uncertainties (see Chapter 5).
-
To design a randomised trial that addresses the agreed most important clinical uncertainties. In line with the Health Technology Assessment brief, we anticipated that the ‘control’ in our randomised trial would be planned vaginal birth and the ‘intervention’ would be planned CS, but this would be informed by the survey.
-
To mock up a short trial protocol (see Chapter 6), together with a rich descriptive vignette of the trial scenario and participant information sheets, to facilitate the qualitative study described below.
-
To perform a qualitative study among clinicians and women to determine remaining key issues and the acceptability of randomisation (see Chapter 7). We aimed to conduct telephone interviews with health-care professionals and focus groups (FGs) with women, including those who have had, or who are at risk of, PTB.
Assuming that there are clinical uncertainties that can be addressed by a trial to which women and clinicians will support recruitment, we aimed to finalise the design (and approximate costs) of a randomised trial of CS compared with vaginal birth to determine the optimal MoB of women presenting in preterm labour.
Chapter 2 Study design
Study design
To achieve our staged aims, we planned two surveys: one with health-care professionals to establish current practice and opinion in key clinical scenarios of women presenting in preterm labour (e.g. cephalic/breech, previous CS or not, growth restriction present or not, gestational age of 23–24, 24–28 or 28–36 weeks) and one with parents to establish current opinion about the best MoB. These surveys are described in more detail, with results, in Chapters 3 and 4.
Informed by the surveys and by an updated literature review, we then convened an interactive working group of clinicians to determine what kind of trials still need to be carried out and what groups of women should be included in these trials. Formal consensus methodology (Delphi) was used to resolve uncertainties. More detail about this Delphi process and the results are described in Chapter 5.
Next, we designed a randomised trial (CASSAVAplus) (see Chapter 6) that would address the agreed most important clinical uncertainties. In line with the Health Technology Assessment brief, the ‘control’ in our randomised trial was vaginal birth and the ‘intervention’ was planned CS, and this approach was validated by the clinician survey. In addition, we generated a rich descriptive vignette of the trial scenario and participant information sheets to facilitate the qualitative study.
Last, we performed a qualitative study among clinicians and women to determine remaining key issues and the acceptability of randomisation. We conducted telephone interviews with health-care professionals, and FGs with women, including those who have had, or who are at risk of, PTB. More details on the qualitative study and its results are provided in Chapter 7.
Thereafter, we had planned to finalise the design (and approximate costs) of a randomised trial of CS compared with vaginal birth to determine the optimal MoB of women presenting in preterm labour. In practice, the COVID-19 pandemic (March 2020) curtailed further project development and so we have been unable to do this. However, Chapter 8 describes strategies for doing this in the future should a trial be commissioned or funded.
Ethics approval and research governance
A study protocol was written to include the surveys, the consensus workshops and Delphi process, and the qualitative interviews and FGs.
The protocol was approved by the London – City & East Research Ethics Committee on 30 April 2019 (reference 18/LO/1616). Local research and development approval was given in Edinburgh on 13 February 2019. Protocol amendments are described in Table 1 and sponsor (University of Edinburgh, Edinburgh, UK) approval was obtained on 15 October 2018, with approval from the NHS Research Scotland Permissions Coordinating Centre and the Health Research Authority on 30 October 2018. The funder approved all versions of the protocol prior to submission to ethics.
| Amendment number | Date of amendment request | Classification | Reason for change | Approved by | Categorya | Approval process complete |
|---|---|---|---|---|---|---|
| 1 | 29 October 2018 | Non-substantial | Funder requested addition of the REC reference number (i.e. 18/LO/1616) and that Section 18 – Protocol Amendments – should state that any amendments should also be submitted to the funder for authorisation prior to submission to ethics | Sponsor, HRA/NRS PCC, REC | C | 29 October 2018 |
| 2 | 25 March 2019 | Substantial 01 | Following the staff pilot survey, we made changes to the CASSAVA questionnaire for staff | Sponsor, HRA/NRS PCC, REC | C | 25 March 2019 |
| 3 | 30 January 2019 | Non-substantial | Change of PI for Edinburgh Royal Infirmary from Professor Jane E Norman to Dr Sarah Stock | Sponsor, NRS PCC | B | 30 January 2019 |
| 4 | 30 April 2019 | Substantial 02 | A new invitation to attend the Delphi meeting to be held in London on 5 July 2019 | Sponsor, HRA/NRS PCC, REC | A | 30 April 2019 |
| 5 | 24 October 2019 | Substantial 03 | New or updated documents:. CASSAVA_ PIS_HCP_V2_17092019. CASSAVA_HCP_Staff_Consent form _ V2_17092019. CASSAVA_PIS (women)_V2_17092019. Cassava_Women_Consent form_V2_17092019. Cassava_Women_OPT-IN_FORM women)_V2_17092019. HCP invitation email to interview_V1_03 Sep 19. CASSAVA_OPT_IN_FORM (HCP)_V1_03092019. CASSAVA Data Information Sheet v1.0_21072018. Social media adverts for CASSAVA focus Groups_V1_03Sep19 | Sponsor, HRA/NRS PCC, REC | A | 24 October 2019 |
| 6 | 16 December 2019 | Non-substantial | One change to the consent form for staff involved in interviews for the qualitative part of the study. The date of the CASSAVA data information sheet was incorrectly noted on the consent form: CASSAVA_HCP_Staff_Consent form _ V3_18 November 2019. CASSAVA Data Information Sheet v1.0_07082018 | Sponsor, HRA/NRS PCC | C | 16 December 2019 |
| 7 | 15 January 2020 | Non-substantial | Typographical correction to the original IRAS form dated 28 August 2018. Name of the hospital site amended | Sponsor, HRA/NRS PCC | B | 15 January 2019 |
| 8 | 15 April 2020 | Non-substantial | Owing to the restrictions imposed in relation to the COVID 19 pandemic, we were unable to arrange for women to attend FGs in person, as planned. It was, therefore, proposed that these meetings be held as virtual FGs in place of face to face interaction. The list of was follows: CASSAVA_PIS (women)_ V3_15042020; Cassava_Women_Consent form_ V3_15042020 | Sponsor, HRA/NRS PCC | C | 4 June 2020 |
Participants
Inclusion criteria
For the survey, we aimed to include consultant obstetricians, neonatologists and midwives working in hospitals with neonatal intensive care units (as these are the only hospitals that will deliver the extreme PTBs, which are included in the scenarios). For the patient survey, we included all parents who responded to the advertisement by Tommy’s (London, UK) and Bliss (London, UK), charities involved in patient support and campaigning for better care for PTB, for responses.
For the consensus workshop and qualitative interviews, we aimed to recruit clinicians (including obstetricians, anaesthetists, midwives, nurses, neonatologists and midwives) with ≥ 5 years experience of providing clinical care to women at risk of preterm labour or preterm infants.
For the consensus workshops and Delphi process, we included women and their partners who fulfilled the following criteria:
-
aged > 16 years
-
willing to consent
-
women with previous experience of preterm labour or delivery
-
women at risk of future preterm labour or delivery.
For the FGs, we recruited women who fulfilled the following criteria:
-
aged > 16 years
-
willing to consent.
Exclusion criteria
For the survey, we excluded clinicians working in units that did not have neonatal intensive care facilities. Women and their partners who experienced adverse events as a result of the issues above (e.g. neonatal death, stillbirth) were not actively excluded from the consensus workshops or FGs, but we were mindful of the need to manage this sensitively. The members of the research team have significant experience of conducting mixed-methods research with parents who have experienced adverse events, including perinatal death.
Recruitment procedure
We aimed to recruit health-care professionals for the survey through our professional networks of contacts. We focused on clinicians participating in existing PTB intervention studies currently led by co-applicants involved in this study. We invited participation through the NHS Preterm Birth Network and Royal College of Obstetricians and Gynaecologists (RCOG) Preterm Birth Clinical Study Group (London, UK). In addition, we planned to advertise the survey through the Royal College of Obstetricians and Gynaecologists, the Royal College of Midwives (London, UK), the British Maternal and Fetal Medicine Society (London, UK), the British Association of Perinatal Medicine (London, UK) and the Neonatal Society (Edinburgh, UK). At the end of the survey, we planned to ask participating clinicians if they would be willing to be contacted to take part in an interview in the qualitative phase of the study.
We aimed to recruit patient participants for the survey through our charity partners Tommy’s and Bliss on an opt-in basis.
Those who agreed to further contact through the survey were invited by e-mail to participate in the consensus working group and Delphi survey.
Clinicians who indicated at the end of the survey that they would be willing to be contacted to take part in an interview were sampled purposefully for an in-depth interview according to their survey responses (ensuring a mix of those in favour of CS and vaginal birth, respectively). Some additional ‘snowball sampling’ was also undertaken to achieve representation of the different kinds of clinical staff who would be involved in recruiting into the proposed trial (e.g. obstetricians, neonatologists, midwives and research midwives) from different areas.
Women were identified for recruitment into the FGs by social media posts on Facebook (URL: www.facebook.com; Facebook, Inc., Menlo Park, CA, USA), Twitter (URL: www.twitter.com; Twitter, Inc., San Francisco, CA, USA) and Instagram (URL: www.instagram.com; Facebook, Inc., Menlo Park, CA, USA), and by charity partner connections with local community groups.
Informed consent
All participants were given an information sheet about the study. Completion of the survey was assumed to indicate consent. Those women/clinicians participating in the Delphi survey, FGs and interviews were asked to provide written consent before they did so.
Outcome measures
Outcome measures were participant opinions, as derived from the surveys, consensus workshops, Delphi, interviews or FGs.
Sample size
Our planned sample sizes were as follows: 200 participants for the clinician survey, 200 participants for the patient survey, 40 participants for the interactive working group and Delphi survey, between 30 and 60 participants for the FGs with women and 25 participants for the interviews with clinicians. No formal sample size calculations were made.
Statistical analyses
Survey data
We used a five-point Likert scale and analysed it with appropriate non-parametric and parametric tests. When > 85% of clinicians responding to the survey agreed on an answer to a clinical scenario, we designated the scenario as having good agreement and being accepted in clinical practice. When < 50% of clinicians agreed on an answer, we assumed that there was significant clinical uncertainty. When 50–70% of clinicians agreed, we designated the scenario as having moderate clinical uncertainty. When 70–85% of clinicians agreed, we designated the scenario as having some clinical uncertainty.
Interactive working group/Delphi survey
Data analysis involved graphical summation of the scores indicating the whole groups’ and individual participant groups’ responses using ‘DelphiManager’ software [www.liverpool.ac.uk/population-health/research/groups/comet-initiative/software/ (accessed October 2021)]. Participants were asked to score each scenario using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) scale for Delphi processes [accessed online: www.gradeworkinggroup.org (accessed October 2021)]. Participants were asked ‘[h]ow important is to include the following scenarios in a randomised trial of mode of delivery (caesarean/vaginal) for a preterm baby?’ A prespecified scale and criteria21 were used for dropping and retaining items in the longlist created from the survey. Scenarios with > 70% of participants scoring 7–10 and scenarios with < 30% of participants scoring 1–3 were included in subsequent rounds.
Qualitative research
Data analysis was undertaken by highly experienced qualitative researchers, with input from other members of the co-investigator team. Individual interviews were read through repeatedly and cross-compared to identify issues and experiences that cut across different accounts. 22 A similar approach was used for the FGs discussions, with particular attention being paid to differences and similarities in the perspectives and views of women belonging to different cultural and religious groups, and to those with prior experience of CS and vaginal birth. In line with recommendations,23–25 careful attention was also paid in the analysis to group interactions, including use of humour, as participants’ (different) assumptions can be revealed through the ways they challenge, question and support one another in the context of a group discussion. 23 Team members undertook separate analyses and wrote independent reports before meeting to discuss their interpretation of the data and reach agreement on key findings and themes, which were then used to inform development of a coding frame. Coded data sets were subjected to further analyses to allow more nuanced interpretations of the data to be developed and to identify illustrative quotations. NVivo software (version 11; QSR International, Warrington, UK) was used to support data coding and retrieval.
Study oversight
A Study Steering Committee was appointed to provide oversight for the study and the members of this committee are listed in the Acknowledgements.
Chapter 3 The clinician survey
Introduction
This chapter describes a survey with clinicians to establish current practices regarding MoB (offering women planned CS or labour with the intention of achieving vaginal birth) and opinions in key clinical scenarios of women presenting at risk of PTB (e.g. cephalic/breech, previous CS or not, indicated birth for pre-eclampsia or not, twin pregnancy or not and 23, 26 or 32 weeks’ gestation). The results were used to inform the subsequent interactive consensus workshop and to provide context to the Delphi exercise.
Methods
We performed a survey of clinicians regarding current practices and opinions, offering examples of key clinical scenarios of women presenting in preterm labour or undergoing planned PTB. The purpose of this survey was to determine the opinions of clinicians (i.e. obstetricians, neonatologists, anaesthetists and midwives working in units with neonatal intensive care facilities) on the optimal MoB in different scenarios.
Prior to designing the survey, we consulted expert opinion at a multidisciplinary conference (the European Spontaneous Preterm Birth Conference, Edinburgh, 16–18 May 2018; ≈ 150 clinicians responded to the survey) to identify scenarios for which there appeared to be most clinical uncertainty. Informed by these discussions, the survey was designed focusing on five scenarios:
-
A woman with a singleton pregnancy with established preterm labour.
-
A woman with experience of a previous caesarean birth and in established preterm labour.
-
A woman with PTB indicated by worsening pre-eclampsia (not in labour).
-
A woman with PTB indicated by fetal growth restriction (not in labour).
-
A woman with twins with established preterm labour.
In each scenario, the clinicians were asked to give their opinion on MoB at different gestational ages (i.e. 23, 26 and 32 weeks’ gestation) and different presentations of baby (i.e. cephalic, flexed breech and footling breech). In each case, clinicians were asked to give their opinion on MoB on a scale from 1 to 7, with 1 being ‘very likely to recommend CS’, 7 being ‘very likely to recommend vaginal delivery’ and 4 indicating ‘equipoise’. There was also an option to indicate that ‘I am uncertain as it is not within my clinical expertise’.
The survey was piloted with 20 clinicians, five of whom repeated the questionnaire 2 weeks later. There was acceptable agreement with responses (75–92%). The wording of questions was modified for clarity in response to feedback.
The questionnaire (see Report Supplementary Material 1) was designed and administered through Jisc online surveys (URL: www.onlinesurveys.ac.uk; formerly Bristol Online Surveys), with options presented as ordinal rating scales. A free-text box for further communication was included at the end of the questionnaire. A request for demographic information of the respondent (including clinical specialty and experience) was included at the end of the survey, along with an option to provide contact details and consent for future contact for interviews and FGs.
The survey was distributed through our professional networks of contacts (including the NHS Preterm Birth Network and RCOG Preterm Birth Clinical Study Group), advertised at meetings (e.g. the British Maternal and Fetal Medicine Society annual meeting, Edinburgh, 28 and 29 March 2019) and promoted on social media.
We acknowledge, a priori, that when surveys are distributed through external organisations it is difficult to determine the proportion of people completing the survey. However, we aimed for completion from around 200 individuals and for representation from different groups of clinicians (e.g. obstetricians, neonatologists, anaesthetists and midwives). This large and diverse sample aimed to overcome any potential bias due to clustering of responses from any particular professional group.
Results were exported in a comma-separated values (CSV) file for analysis. To visualise variation in practice and clinical uncertainty, categories 1 and 2 were collapsed into ‘favours CS’, categories 3–5 were collapsed into ‘uncertain’ and categories 6 and 7 were collapsed into ‘favours vaginal birth’. Results are presented as percentages of clinicians surveyed. When < 50% of clinicians surveyed favoured one MoB (either CS or vaginal birth), then we categorised the scenario as an area of high variation in opinion. When 50–66% of clinicians favoured one MoB, then we classified the scenario as an area with considerable variation in practice. When > 66% of clinicians favoured one MoB, then we classified the scenario as an area with little variation in practice.
Results
We received 224 responses from clinicians working at 72 different UK hospitals and two European hospitals. Details of specialty and experience are included in Table 2.
| Clinician | Total (N = 224), n (%) |
|---|---|
| Specialty | |
| Obstetrics | 114 (50.9) |
| Neonatology | 33 (14.7) |
| Midwifery | 53 (23.7) |
| Anaesthetics | 10 (4.5) |
| Neonatal nursing | 13 (5.8) |
| Other (e.g. radiography) | 1 (0.2) |
| Level of experience | |
| Consultant for ≥ 5 years | 85 (37.9) |
| Consultant for < 5 years | 34 (15.2) |
| Specialty doctor | 5 (2.2) |
| Midwife/neonatal nurse for ≥ 5 years | 58 (25.9) |
| Midwife/neonatal nurse for < 5 years | 14 (6.3) |
| Subspecialty trainee | 5 (2.2) |
| Specialty trainee 4–5 | 8 (3.6) |
| Other (e.g. radiographer) | 1 (0.2) |
The results for each scenario are presented in Figures 1–5 and raw data are included in Report Supplementary Material 2.
Summary of findings for scenarios 1–5
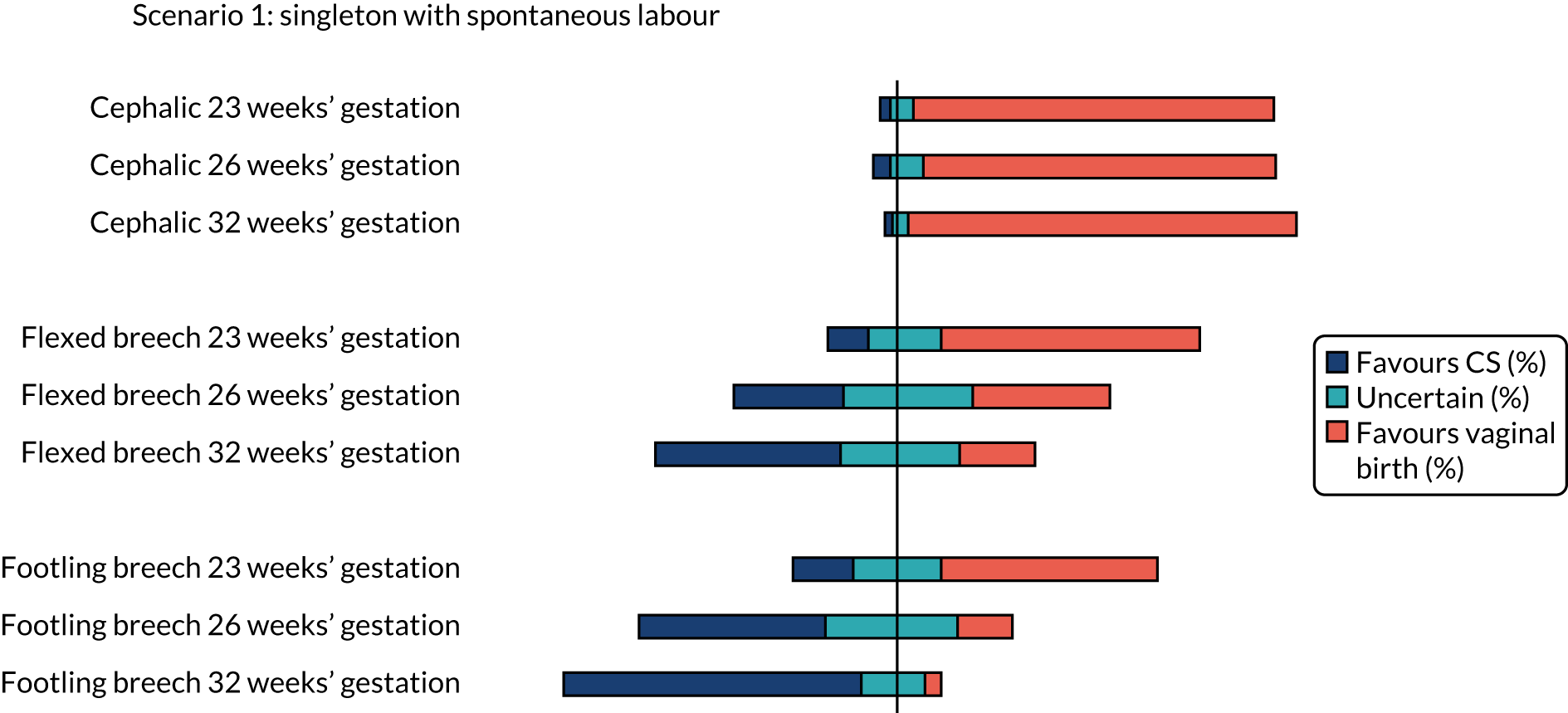
The percentages of clinicians who favoured CS (scores 1 and 2), vaginal birth (scores 6 and 7) or who were uncertain (scores 3–5) on the clinician survey in scenarios relating to a woman with a singleton pregnancy in spontaneous labour are shown in Figure 1. Between 10% and 14% (i.e. between 23 and 31) of the 224 respondents did not feel that the questions on cephalic presentation were in their expertise, 17–18% (i.e. 39 to 41) of respondents did not feel that the questions on flexed breech presentation were in their expertise and 17–20% (i.e. 39 to 45) of respondents did not feel that the questions on footling breech presentation were in their expertise.
FIGURE 1.
Summary of clinician findings for scenario 1.
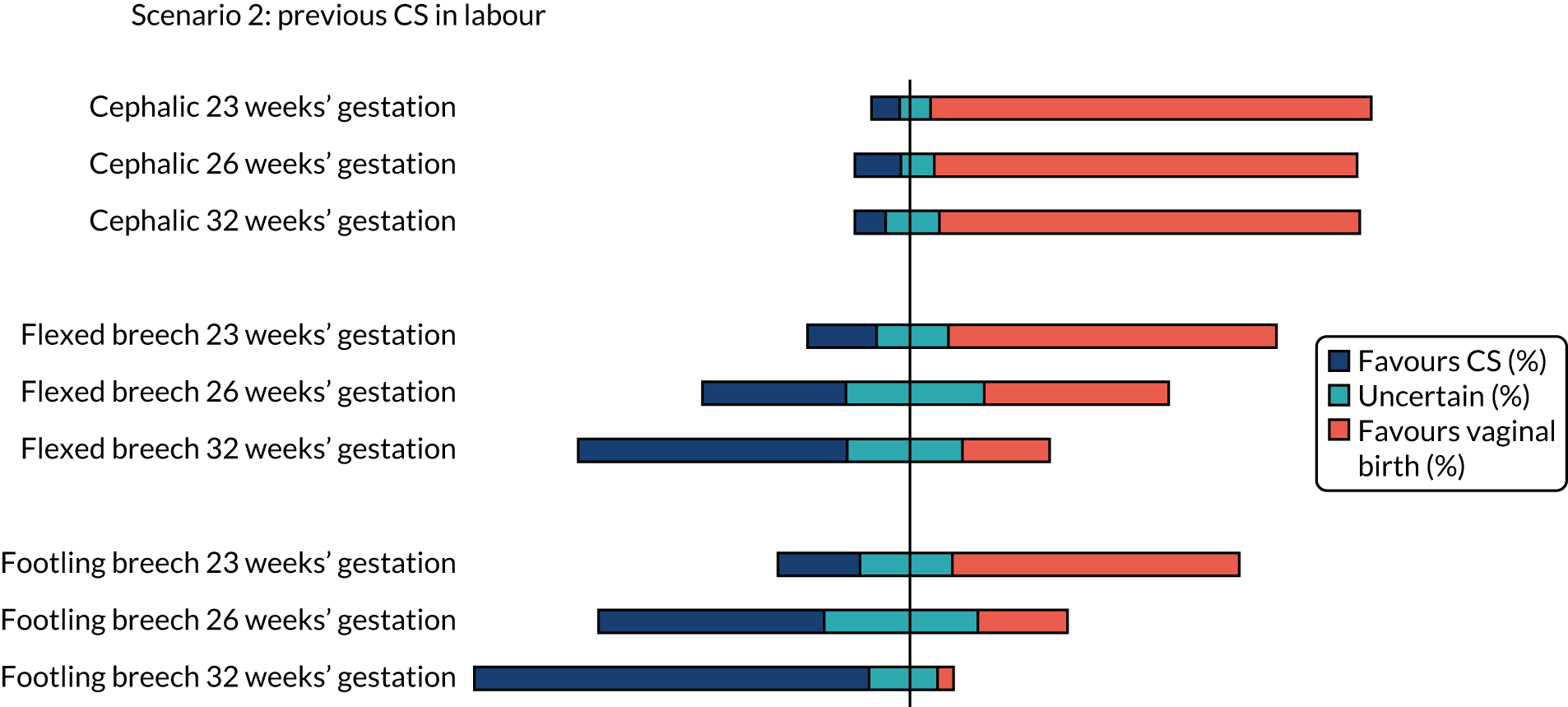
The percentages of clinicians who favoured CS (scores 1 and 2), vaginal birth (scores 6 and 7) or who were uncertain (scores 3–5) on the clinician survey in scenarios relating to a woman with a singleton pregnancy with previous CS in spontaneous labour are shown in Figure 2. Between 12% and 13% (i.e. between 27 and 30) of the 224 respondents did not feel that the questions on cephalic presentation were in their expertise, 18–19% (i.e. 40 to 42) of respondents did not feel that the questions on flexed breech presentation were in their expertise and 17–20% (i.e. 38 to 45) of respondents did not feel that the questions on footling breech presentation were in their expertise.
FIGURE 2.
Summary of clinician findings for scenario 2.
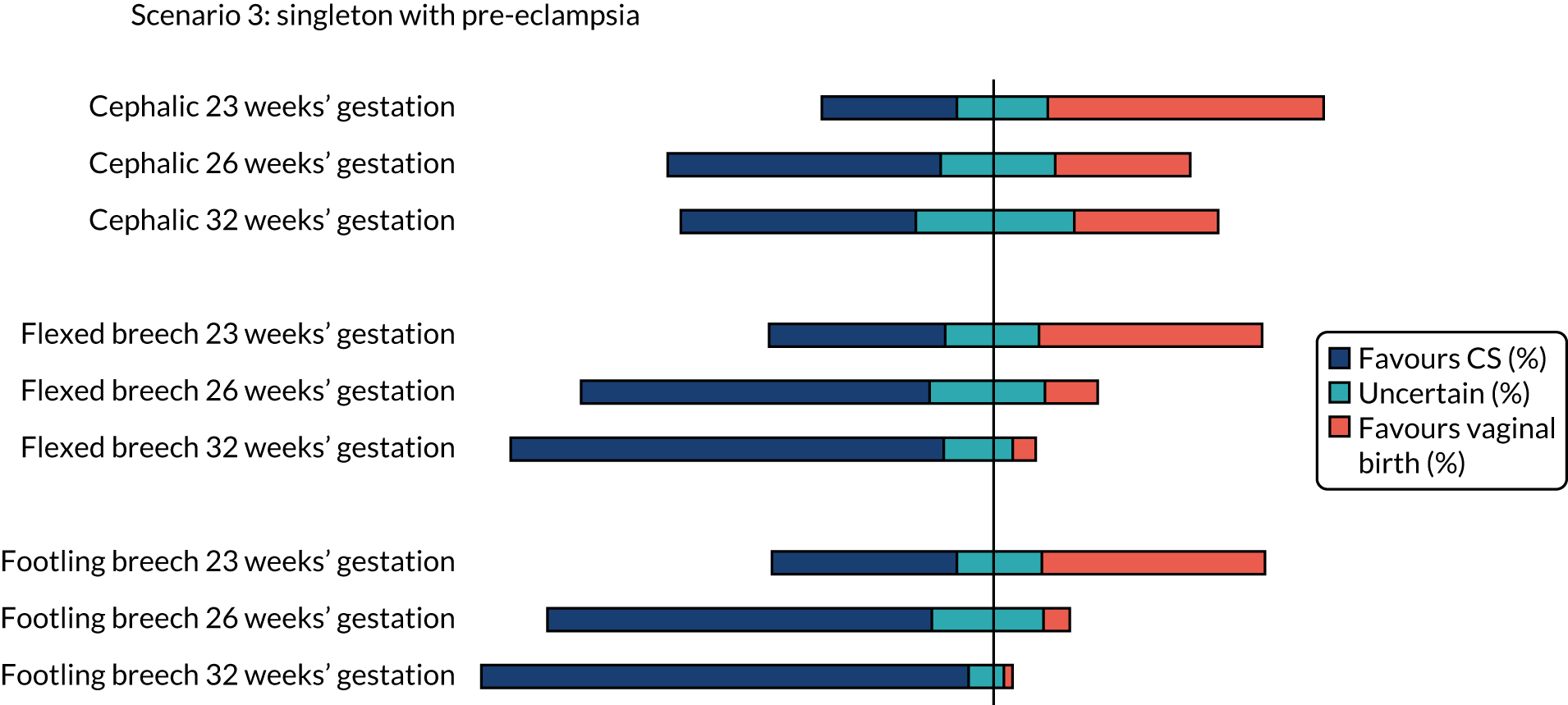
The percentage of clinicians who favoured CS (scores 1 and 2), induction of labour aiming for vaginal birth (scores 6 and 7) or who were uncertain (scores 3–5) on the clinician survey in scenarios relating to a woman with a singleton pregnancy with pre-eclampsia are shown in Figure 3. Between 18% and 24% (i.e. between 41 and 54) of the 224 respondents did not feel that the question on cephalic presentation was in their expertise, 20–25% (i.e. 45 to 55) of respondents did not feel that the question on flexed breech presentation was in their expertise and 19–25% (i.e. 43 to 56) of respondents did not feel that the question on footling breech presentation was in their expertise.
FIGURE 3.
Summary of clinician findings for scenario 3.
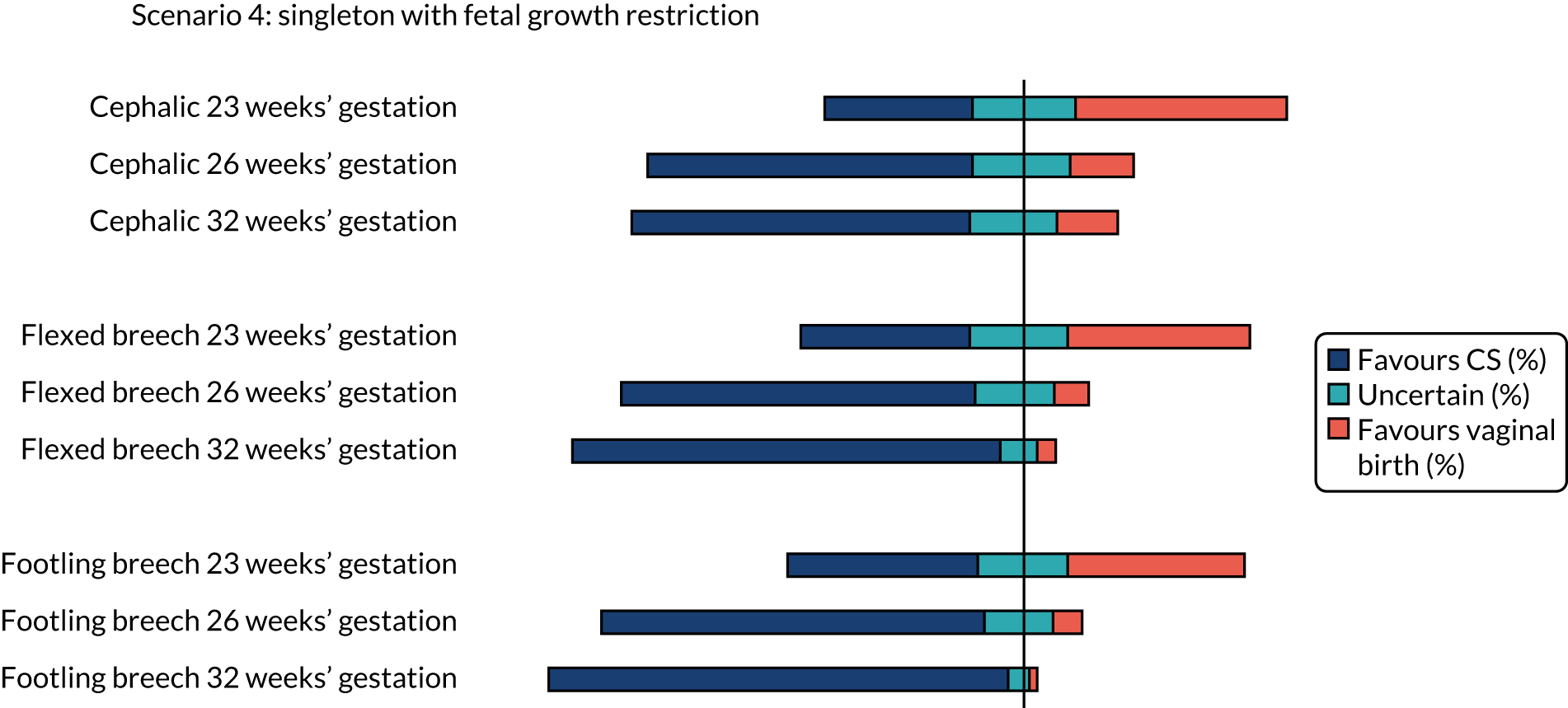
The percentage of clinicians who favoured CS (scores 1 and 2), induction of labour aiming for vaginal birth (scores 6 and 7) or who were uncertain (scores 3–5) on the clinician survey in scenarios relating to a woman with a singleton pregnancy with fetal growth restriction are shown in Figure 4. Between 18% and 22% (i.e. between 40 and 49) of the 224 respondents did not feel that the question on cephalic presentation was in their expertise, 1–24% (i.e. 40 to 54) of respondents did not feel that the question on flexed breech presentation was in their expertise and 17–23% (i.e. 38 to 51) of respondents did not feel that the question on footling breech presentation was in their expertise.
FIGURE 4.
Summary of clinician findings for scenario 4.
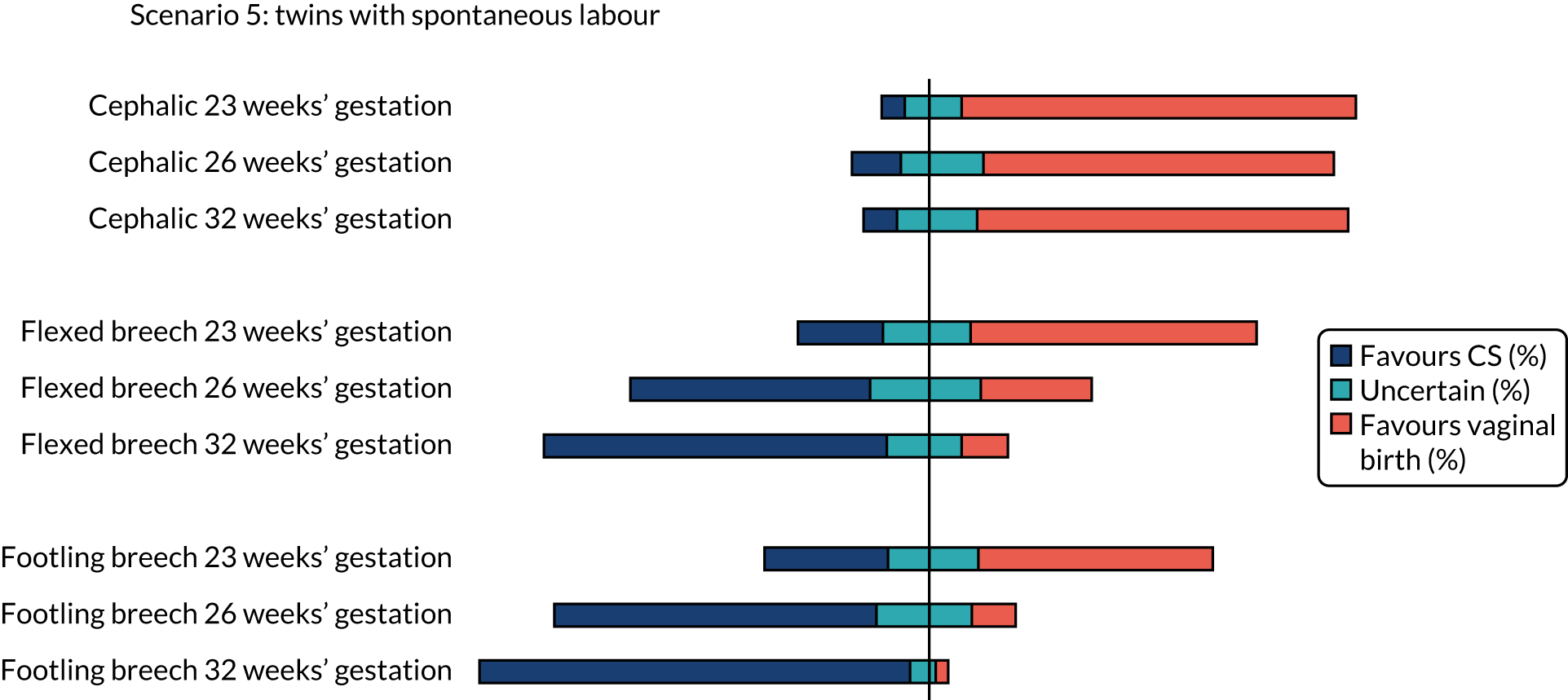
The percentage of clinicians who favoured CS (scores 1 and 2), vaginal birth (scores 6 and 7) or who were uncertain (scores 3–5) on the clinician survey in scenarios relating to a woman with twin pregnancy in spontaneous labour are shown in Figure 5. Between 16% and 18% (i.e. between 36 and 40) of the 224 respondents did not feel that the question on cephalic presentation was in their expertise, 20–21% (i.e. 45 to 47) of respondents did not feel that the question on flexed breech presentation was in their expertise and 19–22% (i.e. 42 to 50) of respondents did not feel that the question on footling breech presentation was in their expertise.
FIGURE 5.
Summary of clinician findings for scenario 5.
Cephalic presentation preterm infant
There was clear consensus for a recommendation of vaginal birth for women in spontaneous preterm labour with a cephalic presentation baby at all gestations assessed (23, 26 and 32 weeks), even with a previous CS or with twins (first twin cephalic) (see Figures 2 and 5). In the scenario of an indicated PTB for pre-eclampsia (see Figure 3) there was less agreement with no consensus at any gestation examined. If the scenario of indicated PTB for fetal growth restriction and a cephalic baby (see Figure 4) there was no consensus on MoB at 23 weeks; but some preference for birth by CS at 26 and 32 weeks.
Flexed breech presentation preterm infant
There was no consensus on recommendations for MoB for women with singleton babies in spontaneous preterm labour with flexed breech infants at 26 and 32 weeks’ gestation (with or without previous CS) (see Figures 1 and 2). At 23 weeks’ gestation, there was still considerable variation in practice, but vaginal birth was the more common recommendation (see Figures 1 and 2). In women requiring an indicated PTB for pre-eclampsia or fetal growth restriction there was no consensus on MoB for babies at 23 weeks’ gestation. However, at 26 weeks’ gestation, there was a slight preference for CS and at 32 weeks’ gestation there was a clear consensus for recommending CS in these scenarios (see Figure 4).
For women with twins in spontaneous preterm labour and first twin flexed breech there was variation, but more clinicians would recommend vaginal birth than CS at 23 weeks’ gestation. At 26 weeks’ gestation, there was no clear consensus. Last, at 32 weeks’ gestation, more clinicians would recommend CS than vaginal birth (see Figure 5).
Footling breech presentation preterm infant
The majority of clinicians would recommend vaginal birth for women with a baby with footling breech presentation in spontaneous preterm labour at 32 weeks’ gestation (regardless of whether or not there was a previous CS and whether or not there was a twin pregnancy, with the first twin presenting in footling breech). At 23 and 26 weeks’ gestation, there was no clear consensus in women with singleton pregnancies and no previous CS. In women who had a previous CS, more clinicians would favour vaginal birth at 23 weeks’ gestation and more clinicians would favour CS at 26 weeks’ gestation. In women with twins in spontaneous labour at 23 weeks’ gestation, there was no clear consensus, but at 26 weeks’ gestation more clinicians favoured CS (see Figure 5).
For women requiring indicated PTB for pre-eclampsia or fetal growth restriction with footling breech presentation, there was no consensus on MoB for babies at 23 weeks’ gestation; however, by 32 weeks’ gestation, there was a clear consensus for recommending CS in both these scenarios. In women with fetal growth restriction, there was also clear preference for birth by CS at 26 weeks’ gestation (see Figure 4). In women with pre-eclampsia, there was more variation, but more clinicians preferred CS than vaginal birth (see Figure 3).
Box 1 summarises the findings on current opinion.
Singleton with spontaneous labour at 23 weeks’ gestation with cephalic presentation.
Singleton with spontaneous labour at 26 weeks’ gestation with cephalic presentation.
Singleton with spontaneous labour at 32 weeks’ gestation with cephalic presentation.
Singleton with spontaneous labour at 23 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour and previous CS at 23 weeks’ gestation with cephalic presentation.
Singleton with spontaneous labour and previous CS at 26 weeks’ gestation with cephalic presentation.
Singleton with spontaneous labour and previous CS at 32 weeks’ gestation with cephalic presentation.
Twins at 23 weeks’ gestation with cephalic first twin presentation.
Twins at 26 weeks’ gestation with cephalic first twin presentation.
Twins at 32 weeks’ gestation with cephalic first twin presentation.
Little variation in opinion: clinicians clearly favour CSSingleton with spontaneous labour at 32 weeks’ gestation with footling breech presentation.
Singleton with spontaneous labour and previous CS at 32 weeks’ gestation with footling breech presentation.
Singleton with pre-eclampsia at 32 weeks’ gestation with flexed breech presentation.
Singleton with pre-eclampsia at 32 weeks’ gestation with footling breech presentation.
Singleton with fetal growth restriction at 32 weeks’ gestation with flexed breech presentation.
Singleton with fetal growth restriction at 26 weeks’ gestation with footling breech presentation.
Singleton with fetal growth restriction at 32 weeks’ gestation with footling breech presentation.
Twins at 32 weeks’ gestation with footling breech first twin presentation.
Considerable variation in opinion: clinicians slightly favour vaginal birthSingleton with spontaneous labour at 23 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour and previous CS at 23 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour and previous CS at 23 weeks’ gestation with footling breech presentation.
Twins at 23 weeks’ gestation with flexed breech first twin presentation.
Considerable variation in opinion: clinicians slightly favour CSSingleton with spontaneous labour and previous CS at 26 weeks’ gestation with footling breech presentation.
Singleton with pre-eclampsia at 26 weeks’ gestation with flexed breech presentation.
Singleton with pre-eclampsia at 26 weeks’ gestation with footling breech presentation.
Singleton with fetal growth restriction at 26 weeks’ gestation with cephalic presentation.
Singleton with fetal growth restriction at 32 weeks’ gestation with cephalic presentation.
Singleton with fetal growth restriction at 26 weeks’ gestation with flexed breech presentation.
Twins at 32 weeks’ gestation with flexed breech first twin presentation.
Twins at 26 weeks’ gestation with footling breech first twin presentation.
High variation in opinion: no consensus among clinicians surveyedSingleton with spontaneous labour at 26 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour at 32 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour at 23 weeks’ gestation with footling breech presentation.
Singleton with spontaneous labour at 26 weeks’ gestation with footling breech presentation.
Singleton with spontaneous labour and previous CS at 26 weeks’ gestation with flexed breech presentation.
Singleton with spontaneous labour and previous CS at 32 weeks’ gestation with flexed breech presentation.
Singleton with pre-eclampsia at 23 weeks’ gestation with cephalic presentation.
Singleton with pre-eclampsia at 26 weeks’ gestation with cephalic presentation.
Singleton with pre-eclampsia at 32 weeks’ gestation with cephalic presentation.
Singleton with pre-eclampsia at 23 weeks’ gestation with flexed breech presentation.
Singleton with pre-eclampsia at 23 weeks’ gestation with footling breech presentation.
Singleton with fetal growth restriction at 23 weeks’ gestation with cephalic presentation.
Singleton with fetal growth restriction at 23 weeks’ gestation with flexed breech presentation.
Singleton with fetal growth restriction at 23 weeks’ gestation with footling breech presentation.
Twins at 26 weeks’ gestation with flexed breech first twin presentation.
Twins at 23 weeks’ gestation with footling breech first twin presentation.
The responses to the question ‘is there a lower limit of gestation before which you would not offer/recommend caesarean section?’ are shown in Table 3. Fifty-four per cent (122/224) of clinicians responded that they did have a lower limit, with the median gestation being 24 weeks (range 21–35 weeks). Thirty-two per cent (71/224) of clinicians had no set lower limit and 14% (31/224) felt that this was outside their expertise.
| Weeks’ gestation | Number (%) of clinicians |
|---|---|
| 21 | 2 (1) |
| 22 | 7 (3) |
| 23 | 28 (13) |
| 24 | 50 (22) |
| 25 | 15 (7) |
| 26 | 16 (7) |
| 28 | 2 (1) |
| 30 | 1 (0.5) |
| 35 | 1 (0.5) |
| No lower limit | 71 (32) |
| Outside expertise | 31 (14) |
Discussion
These findings show that there is considerable variation in UK practice regarding MoB for the preterm infant. However, the findings indicate that in later preterm babies (i.e. 32 weeks’ gestation or more) with malpresentation and/or indicated PTB, there is clear consensus for birth by CS and, therefore, focusing exclusively on these women and infants in a randomised controlled trial (RCT) may result in low recruitment. Similarly, at the extreme preterm gestation of 23 weeks, vaginal birth was generally strongly favoured. The survey suggests most variation in MoB of preterm babies in (1) spontaneous labour with breech presentation between 23 and 32 weeks’ gestation and (2) indicated PTBs (in women not already in preterm labour) with cephalic presentation. These scenarios informed discussions at the interactive consensus workshop and subsequent protocol design.
The survey was aimed to provide context for the subsequent interactive consensus workshop and identify areas where clinical uncertainty was likely. The strengths of the survey were that we surveyed a variety of clinicians from around the UK and that we piloted the survey. Weaknesses are that, although we did check the consistency of responses with a small number of participants (n = 5), there was no formal validation of the survey. Around one-fifth of participants did not feel qualified to respond to different scenarios. However, this information was useful to inform the range of clinician specialties and experience to include in the subsequent qualitative work.
Chapter 4 The public opinion survey
Introduction
This chapter describes a survey with the public to determine their opinions and preferences on MoB for preterm babies. We provided key clinical scenarios of women presenting in preterm labour or undergoing planned PTB and asked patients/the public to indicate their preferred option of vaginal birth or CS. The results were used to inform the subsequent interactive consensus workshop and provide context to the Delphi exercise.
Methods
A survey was designed and placed on Jisc online surveys, which is an online survey tool designed for academic research, education and public sector organisations. A website was set up for the survey and the link distributed, as described below. The survey was designed to be able to be completed on a smart phone or tablet computer, as well as on a laptop or desktop computer. If people did not have computer access, we offered to send a paper questionnaire.
Survey design
The survey asked for basic demographic information (i.e. gender, whether or not the individual had children and whether or not they had experienced PTB). Three clinical scenarios were presented (Table 4). The first clinical scenario was presented for each of the gestations of 23, 26 and 32 weeks. The remaining two scenarios focused on 28 weeks’ gestation only. Respondents were asked to indicate a single option for each scenario and each gestation on a five-point scale as follows:
-
strongly prefer vaginal delivery
-
moderately prefer vaginal delivery
-
no preference for either method of delivery
-
moderately prefer CS
-
strongly prefer CS.
| Scenario number | Description of scenario |
|---|---|
| 1 | Please describe your preferences for the method of delivery if the doctor tells you that a vaginal delivery and a CS are equally safe for you and the baby. (Tick one option for each of the gestations of 23, 26 and 32 weeks) |
| 2 | Please describe your preference for the method of delivery if the doctor tells you that a vaginal delivery is safer for you, and that both CS and vaginal delivery are safe for the baby. You are 28 weeks pregnant |
| 3 | Please describe your preferences for method of delivery if the doctor tells you that CS and vaginal delivery are equally safe for you and that a CS is safer for this baby, but might make a future pregnancy and birth more risky for you and your baby. You are 28 weeks pregnant |
In addition to this ‘tick-box’ question, participants were also asked to provide free-text comments in response to a question ‘[p]lease tell us what else you think the NHS should take into account in deciding whether to recommend a vaginal delivery or a caesarean section in women who are in labour before 37 weeks of pregnancy’.
Survey distribution
The survey was distributed through our charity partners Bliss and Tommy’s in late 2018. The following text was used to advertise the survey. A link to the survey was sent out by each charity, as described below.
Tommy’s advertised the survey on their Facebook page on 20 November 2018. A screenshot of the post is given in Report Supplementary Material 3:
Can you help @EdinburghUni? They’re looking for parents with experience of #prematurebirth to fill in a survey to help them understand the safest way for early babies to be born. To take part in the CASSAVA study or to find out more, go to bit.ly/2S21ICO.
The Tommy’s centre in Edinburgh advertised the survey on their Facebook page on 13 November 2018. A screenshot is given in Report Supplementary Material 3:
We would like to tell you about the CASSAVA study. This study is looking at babies and women who are in premature labour and for whom it is not known which way is the best for them to give birth: whether it is better (safer) by caesarean section (CS) or better (safer) to try and have a vaginal delivery. The first part of this research is to find out where clinicians and women feel that more research on this topic is needed – follow the link to the surveys to help us find out! We would really appreciate your time in filling out the survey appropriate for you. https://edinburgh.onlinesurveys.ac.uk/pilot-cassava-public- . . . (Public Survey). There is also plenty more information on the CASSAVA website www.ed.ac.uk/centre-reproductive-health/cassava Thank you. The Tommy’s team.
Bliss advertised on Facebook, Twitter and Instagram on 7 December 2018. A screenshot of the post is given in Report Supplementary Material 3:
The University of Edinburgh are researching the best way to manage the birth of women presenting in preterm labour. They are inviting parents to participate in an online survey to ask their opinions on the best mode of delivery for preterm labour. You can complete the study at https://edinburgh.onlinesurveys.ac.uk/pilot-cassava-public- . . . If you are interested in learning more about the project you can visit their website at www.ed.ac.uk/centre-reproductive-health/cassava.
By 9 December 2018, 72 people had responded to the survey. Following the Bliss posting, we had 307 further responses. The total number of responders was 379.
The survey closed on 9 January 2019.
Consent
An information sheet was provided to participants before completion of the survey. Those participants who wished to proceed were given access to the survey database. All responders to both the clinician and public surveys were invited to participate in the Delphi questionnaire process (two rounds) by accessing https://delphimanager.liv.ac.uk/Cassava/Delphi, which was active from 1 November 2018 to 9 January 2019 (see Report Supplementary Material 4). All participants who completed both rounds were invited to the consensus meeting in London (held on 5 July 2019).
Results
The summary output from public opinion survey is shown in Report Supplementary Material 5.
A total of 379 people completed the survey, of whom over 95% were female with children and having experienced a PTB. All surveys were completed electronically and there were no requests for completion on paper.
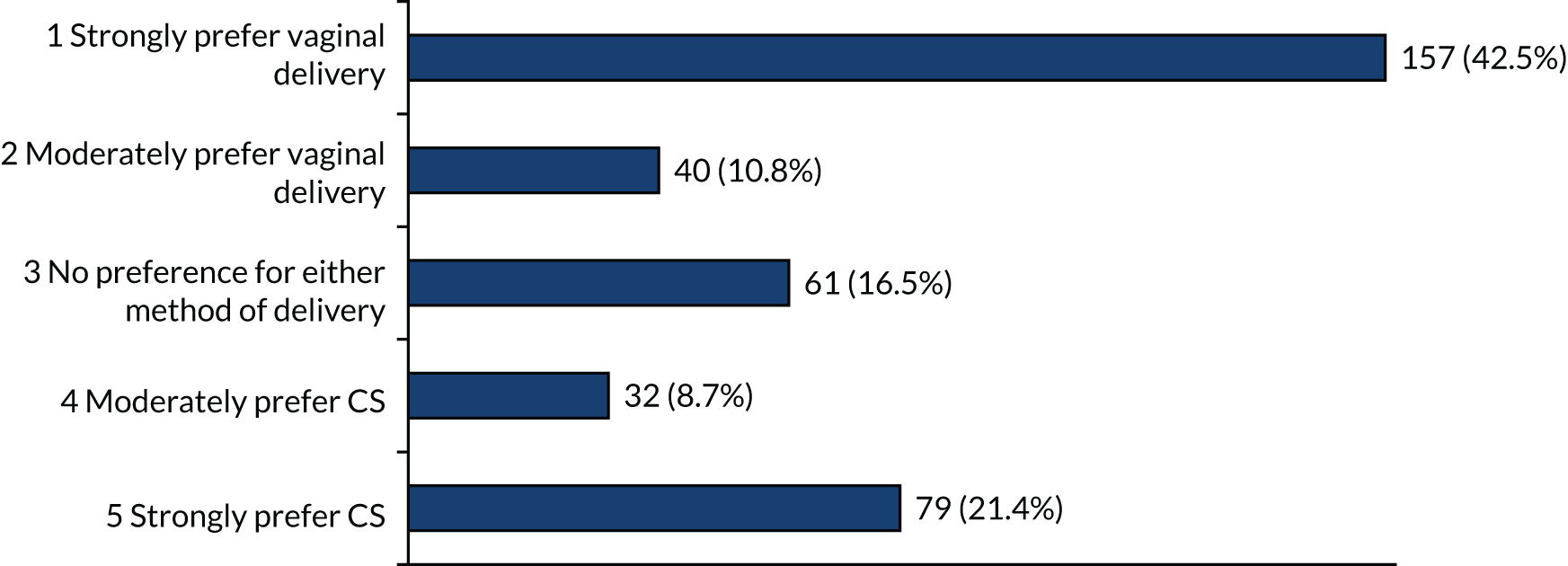
When offered the option of a particular mode of birth, in the scenario with the pregnancy being at 23 or 26 weeks’ gestation, there were strong preferences for vaginal birth (> 40%) and also strong preferences for caesarean birth (> 20%) (Figures 6 and 7). The other options (i.e. moderate or no preferences) were recorded by < 20% of respondents.
FIGURE 6.
Method of birth preferences at 23 weeks’ gestation if each (i.e. vaginal birth and CS) are equally safe for mother and baby.
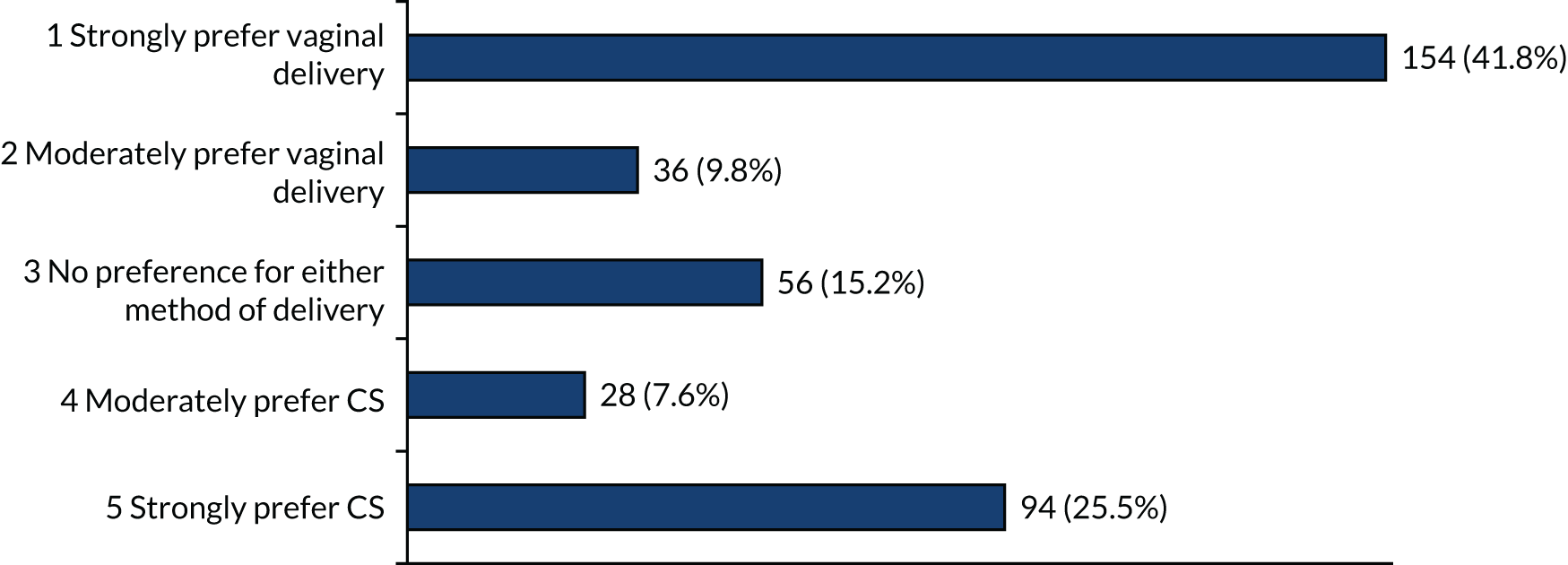
FIGURE 7.
Method of birth preferences at 26 weeks’ gestation if each (i.e. vaginal birth and CS) are equally safe for mother and baby.
For women at 28 and 32 weeks’ gestation, again, there were high proportions of participants with strong preferences for vaginal birth (> 49%) (Figures 8 and 9). Smaller numbers of participants strongly preferred CS at 28 and 32 weeks’ gestation than at earlier gestations (18% at 28 weeks’ gestation and 11% at 32 weeks’ gestation).
FIGURE 8.
Method of birth preferences at 28 weeks’ gestation if each (i.e. vaginal birth and CS) are equally safe for mother and baby.
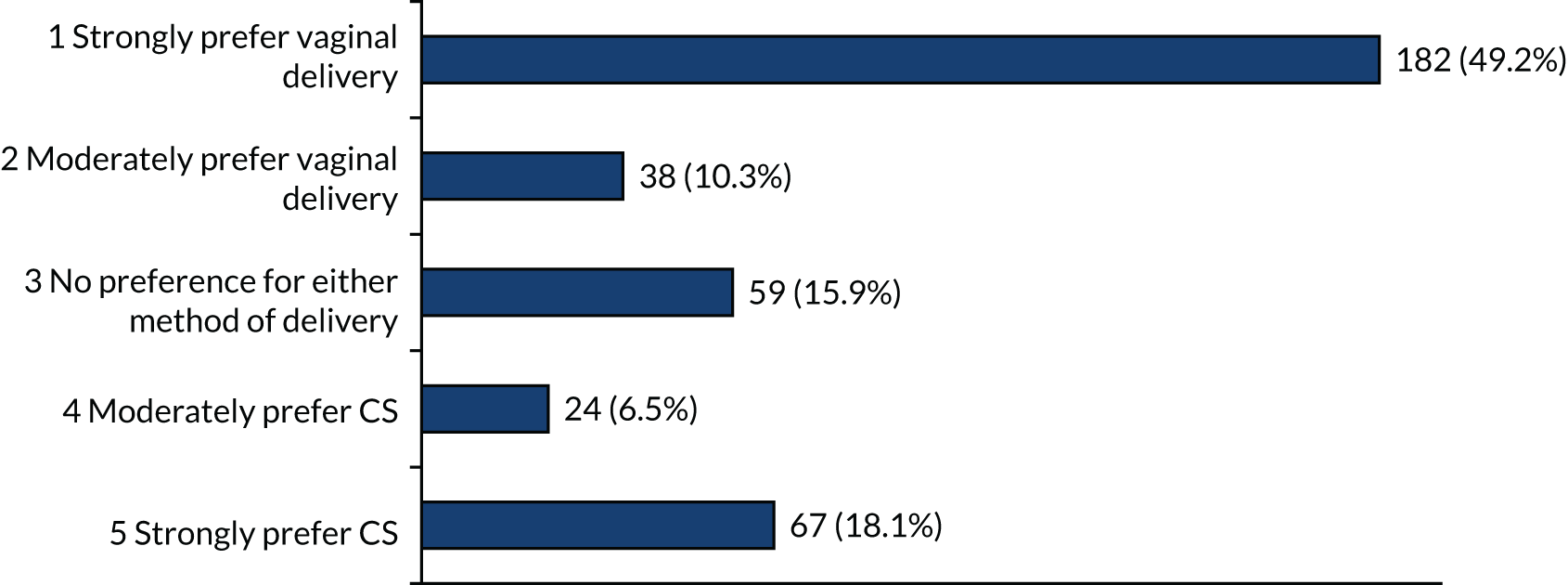
FIGURE 9.
Method of birth preferences at 32 weeks’ gestation if each (i.e. vaginal birth and CS) are equally safe for mother and baby.
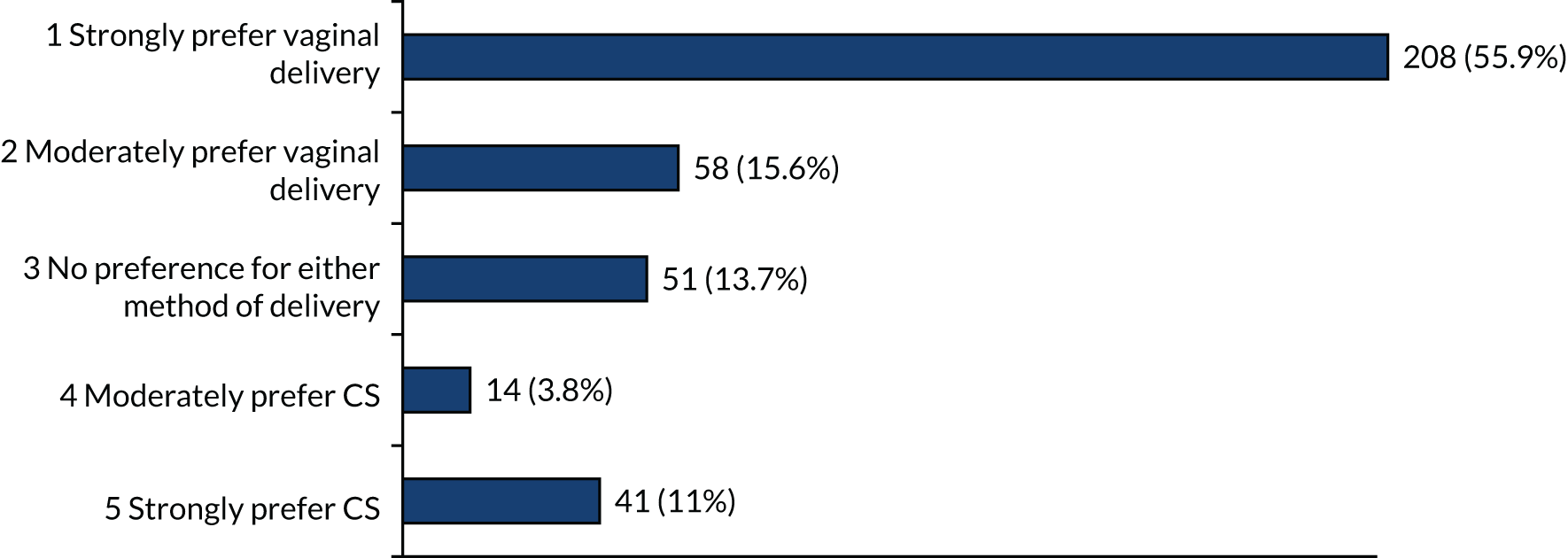
Taken together, these data suggest that individual women have strong preferences for either vaginal birth or CS. Vaginal birth is the most frequent ‘preference’ and becomes more frequent with later gestations (most common at 32 weeks’ gestation). However, a strong preference for CS is most commonly expressed at earlier gestations [most common at 23 weeks’ gestation (26% of women)].
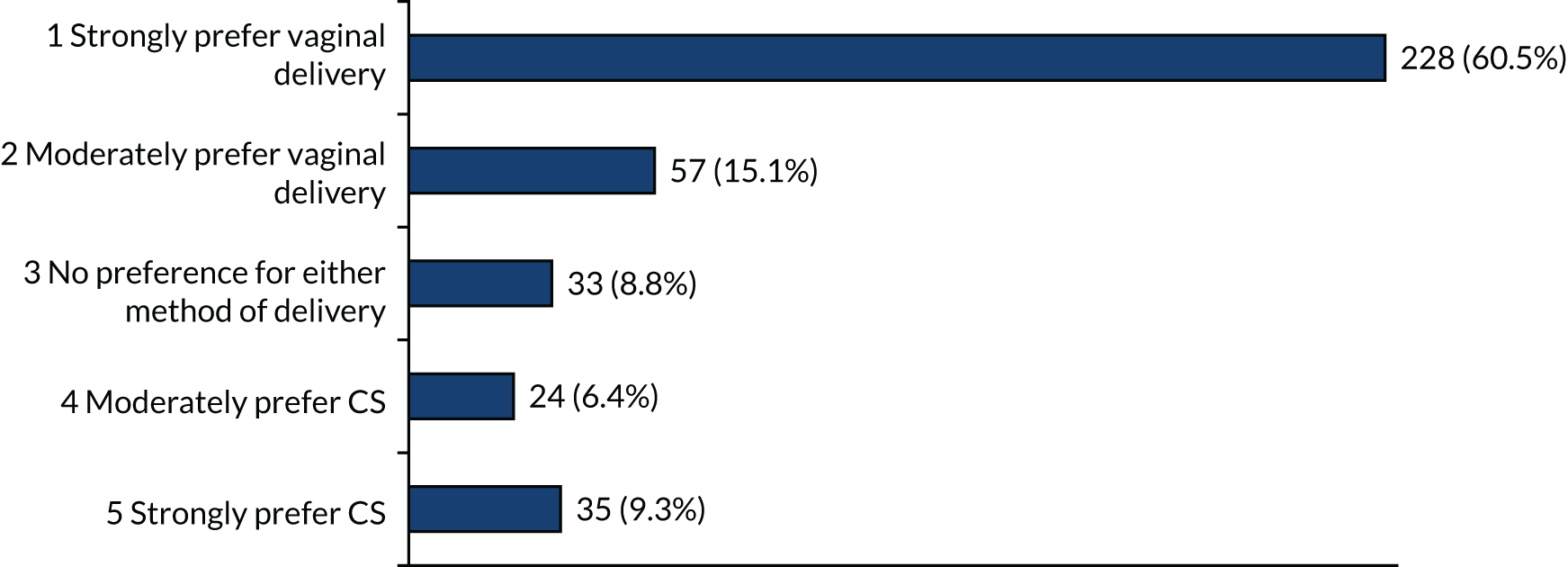
When faced with the scenario that both options are equally safe for the baby and vaginal delivery is safest for mother, there was a strong preference for vaginal delivery (61%) (Figure 10), although 9% of participants still strongly preferred CS.
FIGURE 10.
Method of birth preferences at 28 weeks’ gestation when vaginal delivery is safer for mother and both vaginal delivery and CS are equally safe for the baby.
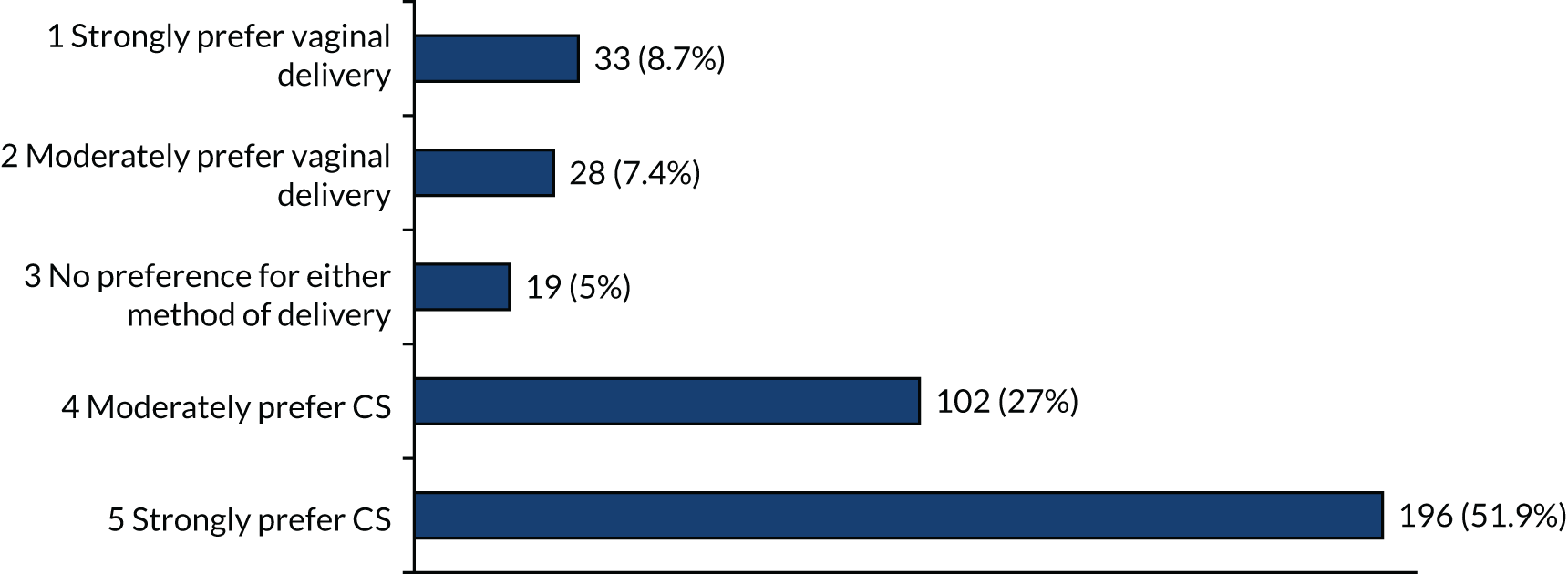
When faced with the scenario that CS is safer for the baby of the index pregnancy, but both CS and vaginal delivery are equally safe for the mother, then 52% of participants report a strong preference for CS and 27% of participants moderately prefer CS (Figure 11). These preferences were described, despite it being acknowledged that CS might make future pregnancies riskier for both mother and baby.
FIGURE 11.
Method of birth preferences at 28 weeks’ gestation when vaginal delivery and CS are equally safe for mother and CS is safer for the baby, but might make a future pregnancy and birth more risky.
Free-text comments are shown in Report Supplementary Material 5.
Key issues mentioned in the free-text comments (in order of frequency) were safety for baby, safety for the mother, recovery times and choice. Presentation of information was also clearly important for respondents.
Discussion
Use of charity partners to access patients and members of the public rapidly generated a large number of responses. Although it is likely that this approach has preferentially selected those who have an interest in this topic, and who have previously engaged with charities focused on this topic, we believe that this approach is likely to be generalisable to the population. It is possible that responses are influenced by people’s own experiences of birth. Importantly, a large majority of respondents had experienced PTB. Although we were able to collect basic demographic information, this did not include information on ethnicity or social exclusion. It is likely that those with limited English, and possibly those from lower socioeconomic groups, are poorly represented in our sample.
Men and women who have not previously experienced PTB were also poorly represented in our sample. Although the decision about MoB is formally made by the pregnant woman, partners, friends and families are influential in women’s decision-making. The demographics of our survey prevents us from knowing whether or not male partners might come to different conclusions and, therefore, alter decision-making in practice.
Many women faced with the decision about how their preterm baby should be born will not have experienced a previous PTB. Including more women for whom this would be a novel experience would have been helpful; however, these women are less likely to be interested in answering a query about PTB and may be less well represented in the contact lists of charities focused (in part) on preventing PTB complications.
The data from our survey suggest the following:
-
Many women are not in equipoise about the best method of birth for a preterm baby and have a strong preference for either vaginal birth (most common) or CS.
-
Women are more likely to choose vaginal birth at later gestations (than at other gestations).
-
A strong preference for CS is more likely to be expressed at very early gestations (than at other gestations).
-
Women prioritise safety of the baby of the index pregnancy ahead of their own safety and against safety of future babies.
These data were used to inform the protocol and the FGs, described in subsequent chapters.
Chapter 5 The Delphi survey
Purpose and rationale
Our aim was to convene an interactive working group of stakeholders to determine what kinds of trials still need to be carried out and which groups of women should be included in these trials.
Formal Delphi consensus methodology was used as a method of systematically collating expert consultation and building constructivist consensus to resolve uncertainties. The Delphi technique is a flexible method and can be adjusted to the respective research aims and purposes, as long as modifications are justified by a rationale and applied systematically and rigorously. There was no intention to arrive at any more than one trial option, but to agree on including or excluding different patient subgroups that could be recruited to a trial. This report follows the recommendations of the CREDES (Conducting and REporting DElphi Studies) guidance.
Planning and design
The multidisciplinary investigator team, including the two co-applicants representing patients and support groups, developed, in an interactive virtual meeting, a longlist of clinical scenarios with equipoise, based on the findings of the surveys described in Chapters 3 and 4 and a systematic literature search completed in May 2019, which identified 54 relevant papers. The scenarios were piloted and edited by the lay members of the investigator team.
In summary, the literature search identified the following prevalent issues: previous caesarean or not (cited in seven papers), number of previous pregnancies (cited in four papers), body mass index (cited in four papers), gestational age/degree of prematurity (cited in four papers), breech presentation (cited in four papers) and multiple (multifetal) pregnancy (cited in four papers).
Other issues identified in the literature included hypertensive disease, gestational diabetes, advancing maternal age, preterm rupture of membranes, chorioamnionitis, placental abnormalities, maternal cardiac conditions, uterine abnormalities, presence of cervical cerclage, fetal growth disorders, cephalic presentation, worrying cardiotocography trace of the fetal heart rate, presentation of twins, previous vaginal birth, previous CS during the second stage of labour (associated with risk of recurrence of preterm labour) and previous PTB. These issues informed decisions by the research team as to which scenarios to include in the rounds, alongside findings from the survey and proposals by participants in round 1.
A two-round three-step Delphi consensus methodology was planned to score and reach consensus on scenarios and subgroups to be included in a future trial. All stakeholder groups, including parents, health-care professionals, researchers and health-care regulators, were invited to participate in the two rounds of the Delphi survey (steps 1 and 2) and a final interactive consensus workshop (step 3).
The aim was for at least 40 participants in round 1 and more than 20 participants in round 2 and the consensus meeting, with at least two or three representatives from each group of participants.
Definition of consensus
A prespecified scale and criteria were used for dropping and retaining items in the longlist.
Scenarios with (1) > 70% of participants scoring 7–10 and (2) < 30% of participants scoring 1–3 were to be prioritised for discussion at the interactive consensus workshop.
Scenarios fulfilling only one of these two criteria were considered borderline.
Study conduct
Informational input
The literature search followed systematic search principles described in the Cochrane handbook. 26 The longlist of scenarios was developed through the clinician and patient surveys.
A summary of the literature and survey findings was presented at the final consensus meeting (i.e. step 3) using Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA).
External validation
The processes and results of all steps were presented to the Study Steering Committee, which made comments and approved the framework for study conduct and interpretation, and, specifically, recommended assessments for attrition bias.
Prevention of bias
To minimise attrition, DelphiManager (i.e. the software used to support the Delphi process) automatically sent reminders every week to registered participants who did not complete each stage and/or had missing data. The investigators did not participate in or influence the reminders.
Attrition bias was estimated by comparing the average scores for different groups of participants between those who completed both rounds and those who completed only round 1 for evidence of significant difference that implied biased participation in the second round as opposed to consensus.
Interpretation and processing of results
Consensus does not necessarily imply the ‘correct’ answer or judgement. Non-consensus and stable disagreement provide informative insights and highlight differences in perspectives concerning the topic in question.
Therefore, we decided a priori to retain all scenarios in both rounds and in all three steps, presenting at the final meeting how scores had evolved over the consensus process, differences between participants groups and the accompanying qualitative comments.
Study process and findings
Expert panel
The Delphi consensus invitees were agreed by the CASSAVA co-investigator team to include UK-based preterm trial leads, members of the RCOG Preterm Birth Clinical Study Group, members of the partners (i.e. Tommy’s and Bliss), other parent organisations (e.g. National Bereavement Care Pathway charities), national stakeholders (e.g. NHS Improvement, NHS England and the Saving Babies’ Lives programme) and all participants in the previous clinician and parent surveys (see Chapters 3 and 4). (Note that this is a more diverse group than described at grant application stage, when we described a Delphi focused on clinicians only, but after reading survey results we felt that it would be helpful to get perspectives from all stakeholders.)
Description of the methods
We used the web-based survey application (DelphiManager) that has been developed by the University of Liverpool (Liverpool, UK) and adopted by the COMET (Core Outcome Measures in Effectiveness Trials) initiative. The web-based Delphi survey is feasible, cost and time efficient and is accepted by users. The survey was hosted within an online portal and infrastructure designed by the University of Liverpool. Before entering the exercise, participants were asked to register, provide demographic details and commit to two rounds.
After the details of participants and the commitment to contribute to two rounds had been reviewed, a unique identifier was allocated. The unique identifier anonymised participant responses but also provided a means to send completion reminders.
Participants were asked to score each scenario using the GRADE scale (accessed online). This scale was originally developed to score the quality of evidence of systematic reviews and has now been adopted in other research studies using Delphi methods (e.g. core outcome sets). Participants were asked ‘[h]ow important is it to include the following scenarios in a randomised trial of mode of birth (caesarean/vaginal) for a preterm baby’.
Free-text comments were also invited and facilitated by DelphiManager.
The Delphi questionnaire was piloted on the Study Management Group, the patient and public involvement (PPI) panel and a sample of stakeholders to ensure the ease of completion by participants prior to recruitment. This also ensured that the scenario terminology was understood by stakeholders before allowing them to decide which scenario was important to them in the Delphi.
In round 1, participants received an e-mail linking to the web-based questionnaire embedded within the study’s website. Initial questions included the option to add additional scenarios for use in round 2 before proceeding to scoring, as additional failsafe steps to identify any scenarios that are prominent with experts and/or parents and should still be considered. Participants scored each scenario listed using the Likert-type scale, as described above. The scores were summarised graphically and indicated the whole groups’ and individual participant groups’ responses that were automatically collated by DelphiManager, using its standard methodology.
After round 1, the data were analysed to produce a summary of results.
It was agreed in an interactive study management meeting that all scenarios would be carried forward to round 2 in addition to scenarios proposed by the participants of round 1 after they had been discussed by the study team and PPI representatives. An anonymous summary of the responses was fed back to participants according to each stakeholder group. Participants were asked to, again, score their preference to reach consensus.
An interactive consensus meeting involving key stakeholders took place following the completion of the Delphi process to consider the scores and agree on inclusions and exclusions, including any scenarios in Delphi round 2 where ‘no consensus’ was found. Only those stakeholders who completed both rounds of the Delphi study were invited to participate to the final consensus meeting.
Procedure framework
Delphi survey rounds (steps 1 and 2)
It was agreed that participants would represent stakeholders in three groups (i.e. the maximum allowed by DelphiManager):
-
parents
-
health-care professionals and researchers (academics)
-
health-care regulators and other stakeholders.
Although, ideally, equal numbers from the three groups would participate, it was agreed that this may not be possible to ensure within the pragmatic constraints of the study. The rules specified that all participants who completed the first two rounds were invited to the interactive workshop. In the lead’s experience, the final workshop was likely to include comparable numbers from the three groups of participants, even though the numbers in each group receiving the original invite to register to the Delphi website may not have been identical.
Each round was agreed to be left open for 2 weeks in view of time constraints. It was deemed essential to complete analysis of the surveys before the workshop and it was expected that analysis would take half a day.
Invitees received an e-mail with an invitation letter that provided a link to the web-based questionnaire. A postal questionnaire was offered but not used by any invitee. All responders to both the clinician and public surveys were invited to participate in the Delphi survey by accessing https://delphimanager.liv.ac.uk/Cassava/Delphi, which was active from 1 November 2018 to 9 January 2019 (see Report Supplementary Material 6). All participants who completed both rounds were invited to the Delphi consensus meeting in London (held on 5 July 2019).
The scenarios were presented in domains (a fixed terminology by DelphiManager that was not possible to amend). Scoring all scenarios on a page was mandatory by default.
The agreed domains, based on analysis of the previous surveys’ findings, were as follows:
-
Domain A [spontaneous PTB with baby flexed breech (the baby is bottom first with its feet right next to its bottom)] at:
-
< 24 weeks’ gestation (A1)
-
24–25+6 weeks’ gestation (A2)
-
26–27+6 weeks’ gestation (A3)
-
28–36 weeks’ gestation (A4).
-
-
Domain B [PTB for obstetric complications (e.g. pre-eclampsia and fetal growth restriction)] at:
-
< 24 weeks’ gestation with any breech presentation (B1)
-
< 24 weeks’ gestation with cephalic presentation (B2)
-
24–25+6 weeks’ gestation with cephalic presentation (B3)
-
26–27+6 weeks’ gestation with cephalic presentation (B4)
-
28–36 weeks’ gestation with cephalic presentation (B5).
-
Following the literature review, additional domains agreed were as follows:
-
Domain C [women with previous caesarean birth(s) in spontaneous labour] at:
-
< 24 weeks’ gestation with spontaneous preterm labour and cephalic presentation (C1)
-
24–25+6 weeks’ gestation with spontaneous preterm labour and cephalic presentation (C2)
-
26–27+6 weeks’ gestation with spontaneous preterm labour and cephalic presentation (C3)
-
28–36 weeks’ gestation with spontaneous preterm labour and cephalic presentation (C4).
-
-
Domain D [women with previous caesarean birth(s) and indicated preterm labour] at:
-
< 24 weeks’ gestation with indicated preterm labour (e.g. pre-eclampsia or growth restriction) (D1)
-
24–25+6 weeks’ gestation with indicated preterm labour (e.g. pre-eclampsia or growth restriction) (D2)
-
26–27+6 weeks’ gestation with indicated preterm labour (e.g. pre-eclampsia or growth restriction) (D3)
-
28–36 weeks’ gestation with indicated preterm labour (e.g. pre-eclampsia or growth restriction) (D4).
-
In round 2, participants were shown the distribution of scores from other participants, along with the score that they attributed to each scenario. Only participants who had scored all scenarios in round 1 were automatically invited by DelphiManager to round 2. Round 2 participants were asked to reflect on their responses, and re-score if they wanted to, having been shown the views of the other participants. DelphiManager facilitates this process by a simple setup followed by inbuilt functionality to calculate the distribution of scores for a particular round. Unlike other online survey tools, the score distribution is then automatically displayed to the participant in the next round, together with a reminder of their own score. An example of what a participant might be shown in round 2 (of a Delphi with a single panel throughout) is shown in Figure 12. Note that scenarios replaced outcomes.
FIGURE 12.
Mock up of Delphi output.
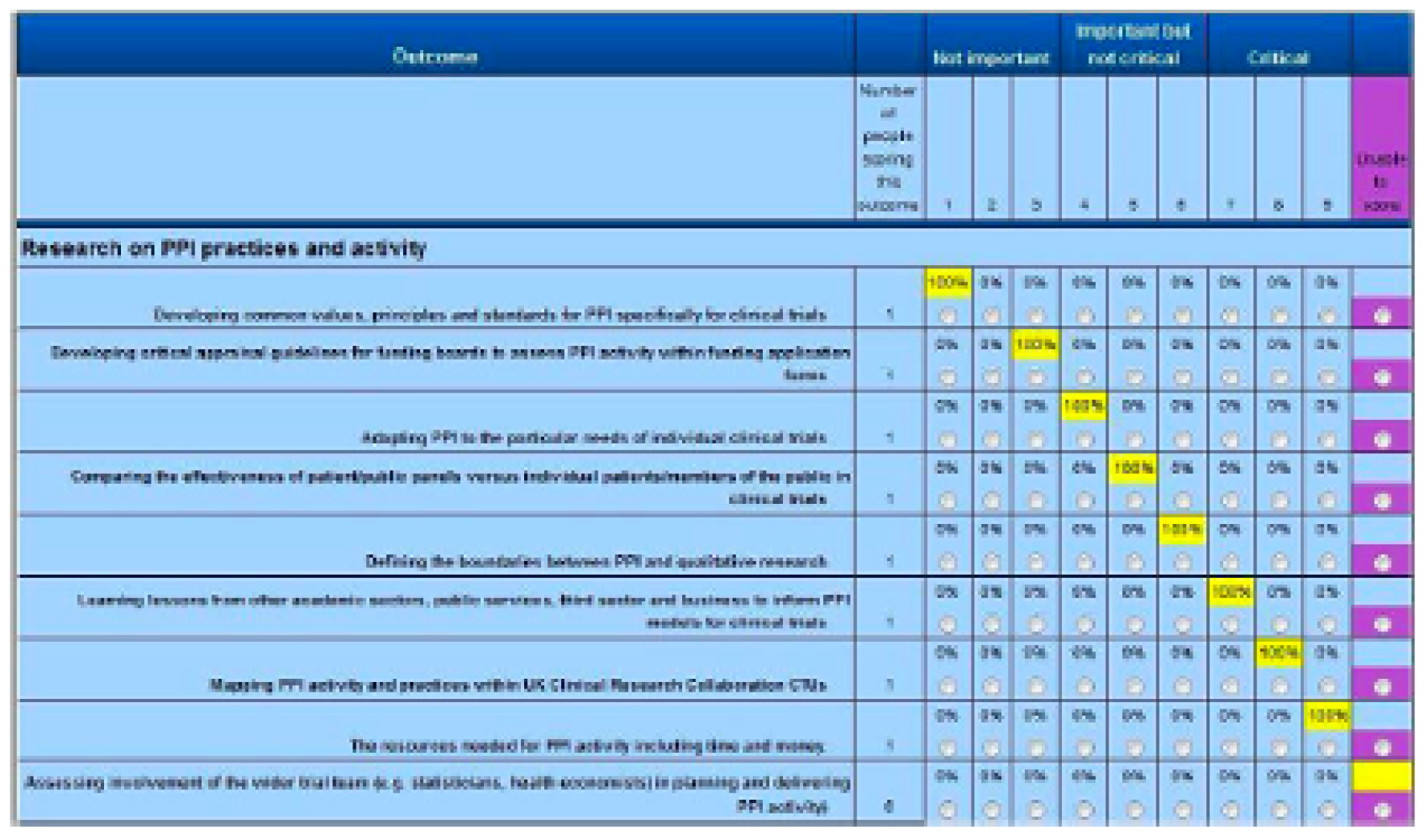
In Figure 12, the participant’s score from the previous round is highlighted in yellow.
For the CASSAVA project, feedback in round 2 was intended to be split according to the three groups described previously. However, it became clear after round 1 that some national stakeholder representatives who had participated had declared themselves as a ‘health-care professional’ as opposed to a ‘stakeholder’. It was decided to amalgamate these two groups, for a final split of (1) parents and (2) health-care professionals/stakeholders.
Results were exported in a CSV file. Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) was used to generate charts.
For statistical tests, we used Stata/SE® 15.1 (StataCorp LP, College Station, TX, USA).
Final consensus: step 3
The final consensus meeting was moderated by the Delphi lead, a clinical lecturer and a PPI representative. As the purpose of this meeting was to elicit scenario(s) that would be desirable and feasible for a trial, which meant the approach had to be inclusive as opposed to highly selective, participants were presented with the results of the two rounds and were invited to discuss each scenario from the final list in detail. Participants were invited to score any final uncertainties using the same scoring scale as in steps 1 and 2. It was intended that the final output would be a couple of inclusive scenarios with clear exclusion criteria (e.g. whether or not spontaneous cephalic labour with no comorbidity should be excluded).
Results
Rounds 1 and 2
Eighty-six participants were included in round 1 (parents, n = 27; health-care professionals, n = 59). Four parents did not score any scenarios and three parents scored only some (but not all) scenarios and were not invited, by DelphiManager, to round 2. Ten parents did not continue after round 1. Ten parents participated in both rounds. One health-care professional did not score any scenarios and two health-care professionals scored only some scenarios and were not invited to round 2. Twenty-eight health-care professionals did not continue after round 1. Twenty-eight health-care professionals participated in both rounds. One new health-care professional who missed the first round was allowed as an exception to join the second round for a total of 29 health-care professionals.
The total number of participants in round 2 was 39 (parents, n = 10; health-care professionals, n = 29), of whom 38 completed both rounds.
After round 1, participants recommended a list of scenarios that were considered by the Study Management Group and PPI representatives (Box 2).
Suspected chorioamnionitis, 22–24 weeks’ gestion.
Suspected chorioamnionitis in labour (> 3 cm), cephalic presentation, > 24–26 weeks’ gestation.
Suspected chorioamnionitis in labour (> 3 cm), cephalic presentation, > 24–26 weeks’ gestation.
Suspected chorioamnionitis in labour (> 3 cm), cephalic presentation, > 28 weeks’ gestation.
Suspected chorioamnionitis, > 24–26 weeks’ gestation.
Suspected chorioamnionitis, 26–28 weeks’ gestation.
Suspected chorioamnionitis, > 28 weeks’ gestation.
Spontaneous PTB with extended breech presentation, < 36 weeks’ gestation.
Indicated PTB with extended breech presentation, < 36 weeks’ gestation.
Admission to NICU in term babies.
Footling breech scenarios.
Abnormal middle cerebral artery doppler studies or ratio.
Previous laser twins, first twin cephalic, spontaneous labour.
Preterm twins with variations on presentation of the first twin, as per previous questions.
Spontaneous rupture of membranes.
Previous obstetric history.
Spontaneous labour.
Induction of labour.
Augmentation of labour.
Spontaneous labour, twin pregnancy, 25–28 weeks’ gestation.
Patient with lethal fetal anomaly at any gestation between 24 and 36 weeks’ gestation.
NICU, neonatal intensive care unit.
Bold text indicates scenarios that were added for round 2 of the Delphi.
The investigator team decided to add four more scenarios to round 2 of the Delphi (see bold text in Box 2). As the DelphiManager website did not allow for the inclusion of additional domains after round 1, it was decided to include the scenarios under domain A.
There was evidence of consensus over the two rounds, with scenarios more likely to be included after round 2 than after round 1 (Figure 13).
FIGURE 13.
Recommended scenarios in Delphi rounds 1 and 2.
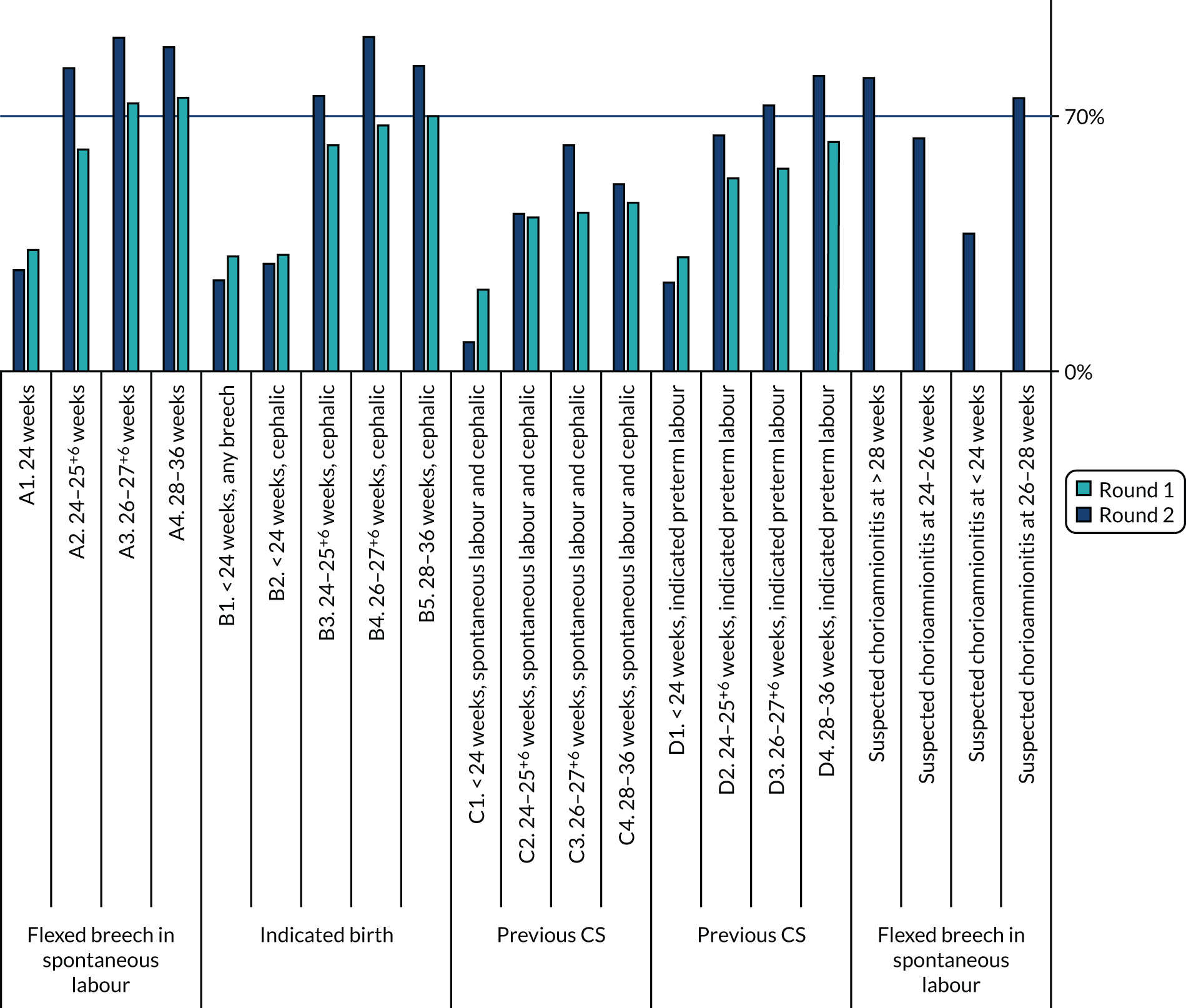
Eighty-one (10.2% of 798 individual scores – 21 scenarios × 38 participants) individual scores were different between rounds 1 and 2.
After round 2, few scenarios fulfilled criteria for exclusion, with most scenarios either qualifying for exclusion or being borderline (Figure 14).
FIGURE 14.
Percentage of participants scoring 1–3 (left axis) vs. 7–9 (right axis).
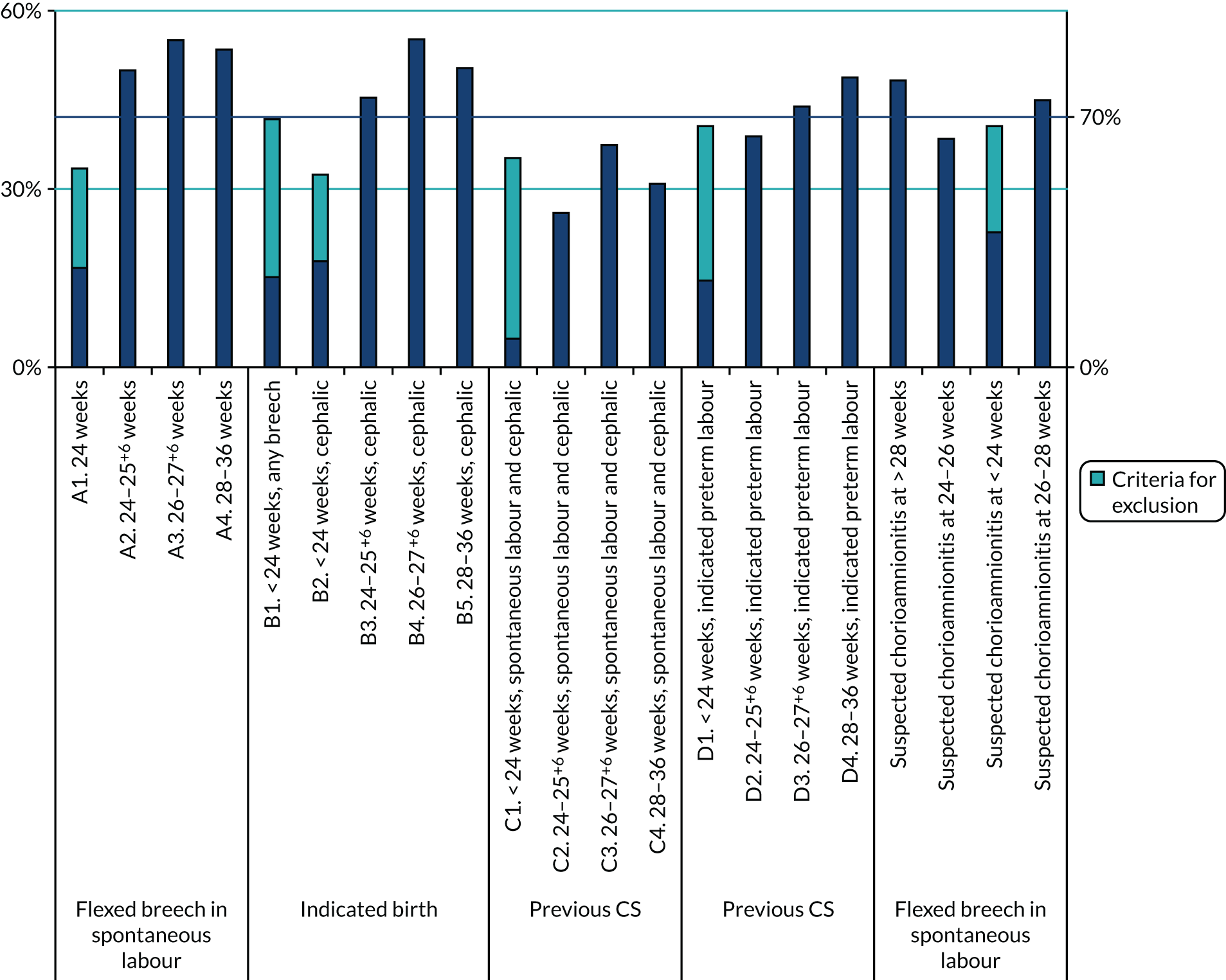
When comparing parents alone with the overall scores, parents were more likely to score borderline scenarios for inclusion in a future trial {e.g. all D scenarios [women with previous caesarean birth(s) and indicated preterm labour]} (Figure 15).
FIGURE 15.
Percentage of parents scoring 7–9 vs. overall scores.
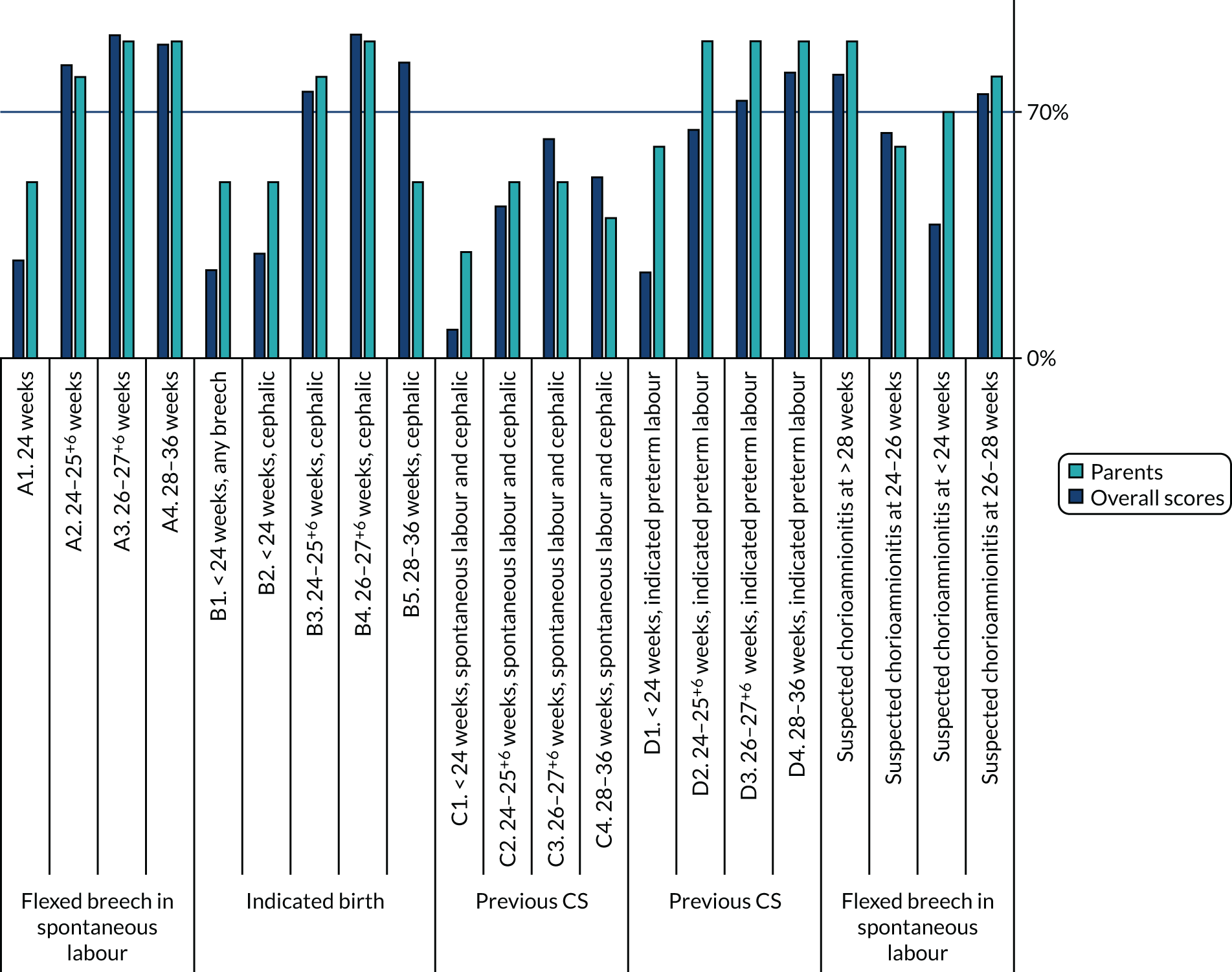
Examples of comments from the Delphi survey rounds are included in Appendix 2.
Attrition bias
Forty participants (n = 12 parents; 30%) completed only round 1 and 38 participants (n = 10 parents; 26%) completed both rounds. One participant completed only round 2.
There was no evidence of significant attrition bias (Figure 16). Scores were not significantly different between those participants who completed both rounds and those who completed only round 1 (Table 5).
FIGURE 16.
Attrition between Delphi rounds.
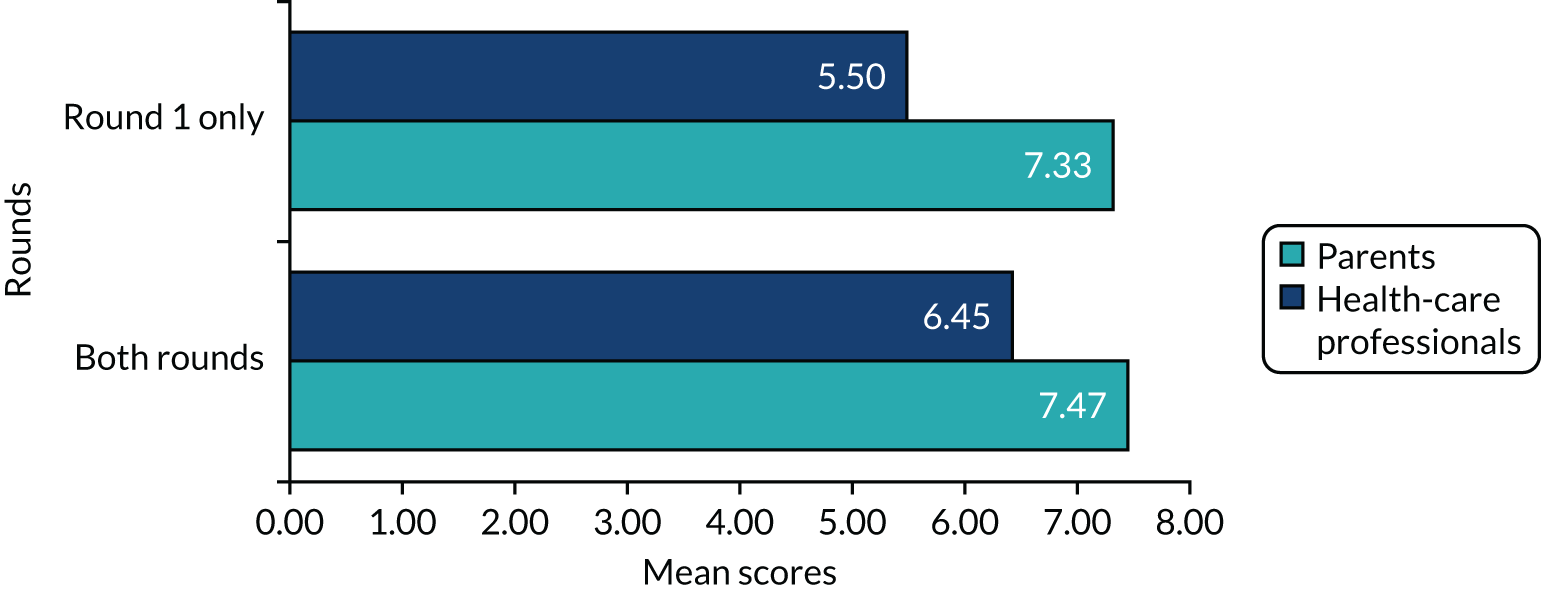
| Participant | Number of participants observed | Mean | SE | SD | 95% CI (p-value) |
|---|---|---|---|---|---|
| Health-care professionals | |||||
| Both rounds | 28 | 6.45 | 0.32 | 1.73 | 5.77 to 7.12 (0.0546) |
| Round 1 only | 30 | 5.5 | 0.35 | 1.94 | 4.77 to 6.22 |
| Parents | |||||
| Both rounds | 10 | 7.47 | 0.41 | 1.32 | 6.52 to 8.41 (0.8062) |
| Round 1 only | 14 | 7.33 | 0.37 | 1.39 | 6.52 to 8.13 |
Consensus workshop (step 3)
Twenty-two experts attended the consensus workshop, including five parents/parent organisation representatives (23%) and two trial managers, as well as one observer.
The areas that required further discussion during the meeting were summarised as follows.
Should the following groups of women be included:
-
Women at ≥ 24 weeks’ gestation with a flexed, footling or extended breech presentation and in spontaneous labour.
-
Women at < 24 weeks’ gestation
-
Women in spontaneous preterm labour with a cephalic presentation.
-
Women who have suspected, or confirmed, chorioamnionitis and cephalic presentation.
-
Women expecting twins.
The group were asked to think about these areas for the discussions. The Delphi survey scores indicated that women at < 24 weeks’ gestation should not be included in the trial. However, the group reflected that this did not demonstrate overall understanding of the purpose of research, which was to improve outcomes, or recognition that neonatal care and survival of babies born at 23 and 22-weeks’ gestation has improved. Parents who participated in the surveys were more inclusive than staff.
Strengths and weaknesses
Besides attrition, the process was prone to the weaknesses of the Delphi process in general, including selection bias (i.e. interested clinicians and patients more likely to participate), language and internet familiarity limitations, and arbitrary consensus criteria.
To optimise the reliability of the findings, we used criteria established and widely used in the literature. We supplemented and enriched the findings of the Delphi with subsequent qualitative research, including with under-represented populations. We calculated and showed that there was no significant attrition bias for professionals and parents alike. Finally, we argue that the purposive sampling technique intentionally recruited those professionals with established interest and expertise, as they are more likely to represent those likely to participate in a future trial as local principal investigators. Parent participants likely represent either potential participants in a trial or lay co-investigators.
Discussion key points
-
All participants agreed that it was difficult to know the right thing to do without evidence, and that how to present this information to women should be explored. It is important to do this well.
-
All participants agreed that the CASSAVA project team would need to look at how to present evidence to women in high-stress situations. Communication is important, as women want confidence in the discussion and clinicians need to be honest in their understanding and experience to help facilitate and agree the pathway of choice.
-
Where possible, it would be preferable to speak to women early in pregnancy or labour. Parents wanted to know about the risks of PTB early, as they felt that it was not included in their antenatal care. Labour is not the best time to present this information, as women are in pain and may have received opiate drugs in labour and (arguably) are not always able to make effective decisions. Antenatal literature should contain information about PTB and birth options should be discussed.
-
The trial design should be the cleanest and clearest design to ensure that women who may benefit are not excluded, including women expecting twins.
-
Does a woman’s obstetric history change what we think about inclusion?
-
Trials should provide capacity for clinicians to randomise at lower gestations (i.e. 22–24 weeks’ gestation).
-
If the situation is life-threatening (e.g. antepartum haemorrhage) then women should not be approached to take part in a trial.
-
Communication is key, especially in the antenatal period. Communication needs to be very clear about possible emergency procedures if the scenario changes and the attending clinician and pregnant woman believe that the randomised mode of delivery is not appropriate.
-
Twins should be included, as trials need to be pragmatic and inclusive.
It was provisionally agreed that women with the following clinical scenarios should also be included in further stages of the CASSAVA project:
-
Women with a baby with flexed or footling breech presentation.
-
Women at > 24 weeks’ gestation with chorioamnionitis and a malposition (e.g. breech).
-
Women with an indicated birth of < 24 weeks’ gestation who have had a previous CS.
-
Women in spontaneous cephalic preterm labour.
Using an online anonymous voting system [URL: www.wooclap.com (accessed 1 September 2021)], the participants scored the following scenarios:
-
Women at ≥ 24 weeks’ gestation with an extended breech presentation and in spontaneous labour (score = 7.2).
-
Women in spontaneous labour at < 24 weeks’ gestation (score = 7.9).
-
Women with chorioamnionitis and a cephalic presentation (score = 7.0).
-
Women expecting twins (score = 7).
(Note that it was agreed a priori that if there was a score of > 5, then there was agreement on balance that the scenario should be included. If there was a score of > 7.5, then there was strong agreement.)
All scores were in favour of allowing these groups to participate.
Additional discussion took place around specific scenarios that are described below.
Extended breech
Generally, the group thought that there would be more equipoise for staff when the gestation was < 30 weeks. However, it was agreed that women in spontaneous labour with an extended breech presentation should be included up to 36+6 weeks. There were also other issues to consider:
-
A competent person needs to be available to perform the CS or a breech vaginal birth.
-
The lack of experience of breech deliveries may be a concern for staff and may result in poor outcomes for the trial and site.
-
Staff would need to provide women with clear explanations, including options to deliver the baby, if complications develop.
-
Is a RCT the right answer for parents? The word ‘random’ has different implications for parents; therefore, will parents be concerned that you are gambling with their babies and their well-being?
Spontaneous labour at < 24 weeks’ gestation
There are improved survival rates and it is challenging to conduct a CS at the lower gestations, as there are risks for the mother and baby. However, the potential benefits for the baby could be greater. The consensus was that we should be as inclusive as possible, with a cut-off of > 22 weeks’ gestation. Review of maternal morbidity rates is required, as there are potentially high risks for the women, especially if only a classical section is available and this would prevent future vaginal deliveries. The following question/issues should be considered:
-
Does a site (centre) have a policy to resuscitate babies at < 24 weeks’ gestation?
-
Classical section competency and information given to women about the risks is required.
-
Ethics considerations of doing this before a baby is legally viable in the UK.
Chorioamnionitis with a cephalic presentation preterm
The group agreed women with chorioamnionitis and a cephalic presentation should be included if in spontaneous labour. The condition must be defined in standard operating procedures.
Using an online anonymous voting system [URL: www.wooclap.com (accessed 1 September 2021)], the workshop participants scored the following scenarios:
-
Women at ≥ 24 weeks’ gestation with an extended breech presentation and in spontaneous labour (score = 7.8).
-
Women in spontaneous labour at < 24 weeks’ gestation (score = 8.0).
-
Women with chorioamnionitis and a cephalic presentation (score = 8.4).
-
Women expecting twins (score = 8.8).
(Note that there were 20 votes, in total, from 22 attendees, as one attendee had left before voting and one attendee could not access the server.)
Items for the trial team to consider are as follows:
-
The protocol needs to clarify study procedures, but be flexible enough to permit local opt-outs.
-
Sites require staff with the skills and experience for breech presentation. Perhaps sites could choose to recruit to the scenario of extended and/or flexed breech, with the ability to opt out of some of the other scenarios.
-
Staff need to be able to explain both options competently, including why they are in equipoise.
-
The planned birth option compared with the spontaneous preterm labour plan should be investigated.
-
Timing between discussion of options and randomisation should be considered.
Strengths and limitations
We acknowledge that participation in the interactive working group was restricted to English-language speakers because of costs. We used FGs to ascertain the views of specific ethnic minority populations that were unable to participate in the working group.
The process was limited by the inflexibility of the Delphi website and software, for example the lack of capacity to add new domains. The support provided for DelphiManager was not 24 hours per day, 7 days per week (24/7), which further limited its flexibility. However, there was excellent participation in all three steps by health-care professionals and parents alike, with no evidence of attrition bias.
Conclusions
The Delphi process was deemed successful. Consensus was reached in the scenarios prioritised in the previous surveys, those added after the literature review and those added after round 1. Parents wanted most scenarios included in further research. Comments by the participants in the two survey rounds (via free text) and the consensus workshop helped provide context to the information input into the next stage (i.e. the qualitative research).
Chapter 6 Development of a short trial protocol
Introduction
A short trial protocol was developed for a trial that we called CASSAVAplus. The purposes of this protocol were specifically to inform a ‘vignette’ for discussion with potential trial participants, and to provide a prototype for discussion with clinicians around recruitment and conduct of a trial. It was not necessarily intended that CASSAVAplus should be the final protocol in any future study.
The trial protocol was designed to determine the best MoB for women and babies in preterm labour. It was developed after the clinician and parent surveys and the Delphi consensus procedure (and was informed by the results of these activities), but before the qualitative work described in the next chapter. The CASSAVAplus protocol was developed by the investigator team (using their expertise and experience in clinical trials for PTB), but without the wider stakeholder discussions that would normally take place in designing a trial. The primary outcome was chosen as one that observational studies have suggested could be affected by mode of PTB and one that would likely affect either clinician or parent choices on MoB. A 5% difference in the rate of primary outcome was agreed by the investigator group as being clinically meaningful and used to calculate the sample size. A fuller version of the CASSAVAplus protocol is described in Appendix 4.
For ease of reference, the ‘PICO’ (population, intervention, control/comparison, outcome) for CASSAVAplus is described below.
Participants
Inclusion criteria for approaching potential participants
-
Women at risk of spontaneous preterm labour, including:
-
women with a previous PTB before 34 weeks’ gestation (spontaneous or induced)
-
women who have had cervical surgery (cone biopsy or cold coagulation)
-
women with a short cervix or positive fetal fibronectin prior to 24 weeks’ gestation.
-
-
Women presenting with signs or symptoms of preterm labour (e.g. contractions, lower abdominal pain, mild per vaginam bleeding, show).
-
Women presenting with preterm premature rupture of membranes.
Or:
-
Women for whom a clinician decision has been made jointly by the clinician and the woman for elective preterm delivery between 22+0 and 36+6 weeks’ gestation.
Exclusion criteria for approaching potential participants
-
Maternal or fetal indications for CS.
Inclusion criteria for randomisation
-
Women 22+0 to 36+6 weeks’ gestation with signs and or symptoms of labour, including:
-
women with cephalic, flexed breech or extended breech presentation
-
women with a live baby in whom it is anticipated resuscitation will be attempted.
-
Or:
-
Women 22+0 to 36+6 weeks’ gestation in whom elective preterm delivery will be performed in the next 72 hours, including:
-
women with cephalic, flexed breech or extended breech presentation
-
women with a live baby in whom resuscitation will be attempted.
-
Exclusion criteria for randomisation
-
Triplets and higher-order multiples.
-
Diagnosed intrauterine death.
-
Advanced labour, such that CS cannot be performed safely (at clinician’s discretion).
Intervention
The intervention was CS.
Comparator
The comparator was vaginal birth.
Outcomes/end points
Primary outcome
Neonatal composite of alive at 6 months after birth or to home discharge (whichever is sooner) and without significant IVH (defined as grade 3 or 4 IVH) or cystic periventricular leukomalacia.
Secondary outcomes
Maternal: major maternal morbidity (as a composite and as individual components)
-
Post-partum haemorrhage ≥ 1 litre.
-
Admission to the main intensive care unit for > 24 hours.
-
Bowel injury requiring repair.
-
Bladder injury requiring catheterisation for > 48 hours.
-
Other organ damage.
-
Postnatal anaemia < 70 g/dl (or requiring a transfusion).
-
Postnatal infection (sepsis) requiring intravenous antibiotics for > 48 hours.
-
Anal sphincter injury.
-
Postnatal depression requiring inpatient admission.
-
Hysterectomy.
-
Return to theatre for any other reason.
-
Confirmed thromboembolic disease.
-
Post-traumatic stress disorder.
-
Hospital stay of ≥ 7 days.
Neonatal
-
Incidence of Apgar scores of < 7 at 1 and 5 minutes.
-
Early mortality (i.e. < 72 hours of life).
-
Mortality before 6 months of age/discharge home alive.
-
Significant IVH (defined as grade 3 or 4 IVH).
-
Cystic periventricular leukomalacia.
Costs
-
Costs of major maternal morbidities from recruitment to 6 months postnatally.
-
Costs of major neonatal morbidities from recruitment to 6 months postnatally.
A fuller description and a flow chart of CASSAVAplus is shown in Appendix 3, Figure 17.
Chapter 7 Qualitative research
Introduction
As noted earlier, the research evidence3,4 on which health-care professionals and/or women make their decisions with regard to MoB in PTB is inconclusive. Considerable effort has been made to resolve the resulting clinical uncertainties, with at least six RCTs attempted to date. 3 However, all those trials were stopped early, reporting difficulties with recruitment (collectively enrolling just 122 women). Although limited information is available on why recruitment to those trials proved so challenging, health-care professionals’ reluctance to randomise eligible women was reported as being an important factor in some instances. 3 Survey data from a study exploring willingness to participate in a hypothetical trial suggests that pregnant women may be similarly reluctant to enrol,27 although trials comparing MoB at term have recruited adequately, albeit with the involvement of many sites (around 120) in many (25+) countries. 13,18 The success of those trials suggests that, with appropriate design and resourcing, MoB trials are feasible, which is a view also expressed by Alfirevic et al. 3
Over recent years, qualitative research, drawing on the perspectives of both health-care professionals and patients, has played an increasingly important part in progressing understanding of why clinical trials succeed (or fail). For example, such research has shown how lack of or unstable equipoise (Box 3) regarding trial interventions may affect health-care professionals’ engagement. 30–32 Qualitative research has also highlighted how more pragmatic resource-related concerns (shortfalls) may undermine even committed health-care professionals’ support for trials. 33 Research involving patients has provided further insight into the barriers to and facilitators of trial recruitment more generally and in particular specialisms/areas. 34–36
The term equipoise refers to a situation where clinicians and/or patients are unable to determine the superiority of, and have no preference between, different interventions or care options. 28 Clinical equipoise, sometimes referred to as ‘community equipoise’, is said to exist when the medical profession collectively agrees that insufficient evidence exists to determine the superiority of one treatment option over another. 29 A RCT can be justified (only) when clinical equipoise exists. However, although clinical equipoise may provide the driving force behind a RCT, individual practitioners may hold competing views and have preferences for care that are at odds with the collective viewpoint (i.e. they may lack individual or personal equipoise). The degree to which individuals are uncertain about the effects of interventions may vary and, to complicate matters further, it is possible to be uncertain but still not be in equipoise. 30
Given the potential challenges of recruiting into a trial of MoB in PTB, and to help inform decisions about whether or not such a trial should go ahead in the future, our qualitative study sought to address the following aims.
Aims
-
To determine (1) whether or not health-care professionals would be willing to recruit women into the proposed trial and (2) whether or not women would be willing to participate.
-
To explore if (and what) aspects of the trial’s design would need to change to make it more acceptable to health-care professionals and women.
-
To determine whether or not there are any practical, logistical or other challenges likely to impede trial delivery, and establish what resourcing and other support health-care professionals might need to overcome these.
-
To understand and explore the potentially distinctive challenges of recruiting ethnic minority women into the trial, and to establish any additional resourcing and support needed to optimise these women’s participation.
Although we were successful in delivering aims 1–3, for reasons detailed below (specifically, the impact of the COVID-19 pandemic on data collection activity) we were able to address aim 4 less satisfactorily.
Methods
Overview
The qualitative substudy featured an emergent, inductive design, involving an iterative and reflexive approach to sampling, data collection and analysis. Two methods were employed: (1) semistructured interviews to solicit health-care professionals’ perspectives on the hypothetical trial and (2) FGs to gather the views of women. Data collection was undertaken in the UK from October 2019 to March 2020 when research activity (i.e. data collection) was suspended by the sponsor because of the COVID-19 pandemic. We provide a detailed description of our methods, in accordance with COREQ (COnsolidated criteria for REporting Qualitative research),37 below.
Interviews with health-care professionals
Interviews with health-care professionals explored health-care professionals’ views about a hypothetical trial protocol (HTP), which was developed by the co-investigator team in the light of findings from earlier phases of the study, as described in Chapter 6. Two versions of this HTP (i.e. summary and full) were produced (see Appendices 2 and 3), with both written in a style familiar to health-care professionals. We aimed originally to discuss the HTP with approximately 25 health-care professionals; however, when research activity was suspended, we had completed 24 interviews. We are reasonably confident that data saturation was reached in these health-care professional interviews.
Sampling: health-care professionals
A purposive sampling strategy was discussed and agreed with the co-investigator team. The goal of the purposive sampling strategy was to capture the perspectives of health-care professionals who had experiences relevant to the HTP and who had worked in a range of roles and maternity settings across the UK. As the HTP included PTB at lower gestations (down to 22 weeks’ gestation), we prioritised health-care professionals working in, or with experience of, settings providing level 3 neonatal care (i.e. offering an extended period of intensive care to neonates, e.g. because of PTB at < 27 weeks). Given that obstetricians are key to the recruitment of women into intrapartum trials, a decision was made to ensure good representation of this professional group. We also decided to include neonatologists, given their role in counselling parents (in particular, about neonatal care) prior to PTB. As midwives play a role in supporting women at risk of and/or during PTB,38 and research midwives play an important part in the delivery of trials,39 our sampling strategy was further designed to include both clinical midwives (working on labour wards/emergency admission units) and research midwives. We aimed to involve health-care professionals of all types and with varying lengths of clinical experience and levels of responsibility.
Recruitment: health-care professionals
Health-care professionals completing the phase 1 survey were asked to indicate their willingness to be contacted about participation in a telephone interview. Seventy-eight individuals working in a variety of roles agreed to this and the CASSAVA study administrator e-mailed selected respondents an invitation to take part in an interview. Attachments to the e-mail included a participant information sheet and an opt-in form that were returnable directly to the qualitative research team.
In addition to approaching health-care professionals responding to the phase 1 survey, and as proposed in our original grant application, the qualitative research team identified and recruited other potential study participants using snowballing techniques. This approach involved asking interviewees to forward study information to colleagues who met our sampling criteria and might, potentially, shed light on emergent issues. 40,41 A breakdown of health-care professionals recruited through each of the two routes is provided in Table 6.
| Recruitment phase | Number of health-care professionals recruited |
|---|---|
| Recruitment from phase 1 survey | |
| Number of health-care professionals from phase 1 survey indicating willingness to be contacted regarding interview | 78 |
| Number of health-care professionals from phase 1 survey currently practising in settings with level 3 neonatal provision | 56 |
| Obstetricians | 44 |
| Neonatologists | 5 |
| Midwives | 7 |
| Number of health-care professionals from phase 1 survey practising in settings with level 3 provision and agreeing, in principle, to interview | 13 |
| Obstetricians | 12 |
| Neonatologists | 1 |
| Midwives | 0 |
| Number of health-care professionals from phase 1 survey who ultimately took part in an interview | 10 |
| Obstetricians | 9 |
| Neonatologists | 1 |
| Midwives | 0 |
| Recruitment via snowballing | |
| Number of health-care professionals identified via snowballing who took part in an interview | 14 |
| Obstetricians | 3 |
| Neonatologists | 4 |
| Midwives | 7 |
Data collection
Health-care professionals who agreed to take part in an interview were sent information about the hypothetical trial prior to the interview. This information took the form of a three-page summary document (see Appendix 3), which they were told would form the focus of the interviews. Health-care professionals were also sent a more detailed 14-page document (see Appendix 4) that they were invited to consult if clarification was needed. All interviews were undertaken by Sushila Chowdhry, a social scientist with a background in health care. The interviews took the form of a ‘guided conversation’,42 loosely following a topic guide (see Report Supplementary Material 7) focused on key features of the HTP. The topic guide was developed with input from the co-investigator team and informed by the wider (trials) literature. It was revised as data collection progressed, in keeping with the inductive approach utilised in the study. 43 For example, we had originally intended to explore, with health-care professionals, the potentially distinctive challenges of recruiting ethnic minority women into the trial (and any resourcing and support that might mitigate this). However, this area of enquiry was de-prioritised because of the complexity of the trial design we were tasked with exploring with health-care professionals, health-care professionals’ highly qualified support for the proposed trial and the limited time most interviewees could commit. Therefore, a pragmatic decision was made, relatively early on in data collection, to focus interviews on key elements of the trial design. Interviews were conducted by telephone, between October 2019 and March 2020, at times convenient to participants. The interviews lasted 30–64 minutes and informed consent was obtained.
Data analysis: health-care professional interviews
All interviews were transcribed verbatim by a professional transcription company. Our approach to data analysis observed the principles of inductive analysis and involved ‘discovering patterns, themes, and categories in one’s data’. 43 To achieve this, transcripts were read and re-read by three members of the qualitative team (SC, RIH and JL), and emergent patterns and ideas were discussed before the interview data were systematically coded and codes sorted into themes. 44 Sushila Chowdhry and Ruth I Hart then produced detailed analytical reports, which provided a basis for discussion with the wider qualitative research team and were used in the writing of this final report. Team discussions considered areas of convergence and divergence, providing a catalyst for further analysis and increasing the dependability of the final analysis. 45 This collaborative and dialogic approach encouraged reflexivity (i.e. attention to how personal values and assumptions may have had an impact on research activity and data interpretation). Qualitative software (NVivo version 11) was used to facilitate data coding and retrieval.
Focus groups with women
We aimed originally to undertake six to eight FGs, involving women with diverse pregnancy experiences and including three groups dedicated to ethnic minority women. Regrettably, only our pilot group (PG) plus two actual FG discussions (FGs 1 and 2) had been conducted when research activity was suspended in March 2020. Further groups, which were in advanced stage of organisation, had to be cancelled (see below for more information about the completed and planned groups).
Consultation to see if virtual groups would be acceptable
As some of the women from the cancelled groups suggested doing FGs using virtual methods, we submitted a proposal to the sponsor (on 15 April 2020) to conduct the remaining groups virtually (i.e. online). The sponsor assessed our submission as a (non-substantial) amendment and advised that further ethics/governance approval would be required. Alongside this, we liaised with Bliss’ Insight and Involvement Group (made up of a broad range of parents with neonatal experience) to seek members’ perspectives on the idea of conducting virtual FGs. Sixteen women offered feedback. Although these women were supportive of the idea of FGs being carried out virtually, they also raised significant concerns. Specifically, there were some worries that participants, especially those currently pregnant, might be experiencing heightened anxiety due to the pandemic, which could potentially be compounded by research participation. It was noted that should a woman become distressed during a virtual FG the facilitator would be unable to take her to a private space and offer comfort and information. These concerns led the qualitative and wider co-investigator group to conclude that it would be ethically problematic to collect data using virtual methods. Data collection was, therefore, discontinued.
Sampling: women
Guided by discussions with our PPI group (organised through the CASSAVA project’s partner organisations and the wider co-investigator team), we developed a detailed sampling strategy. To elicit a diversity of perspectives on the hypothetical trial, we aimed to sample for women who had previously experienced either spontaneous or planned PTB; women who were contemplating pregnancy, or were currently pregnant, and judged low risk for PTB; women who were pregnant and considered at high risk of PTB; and women who had experienced elective and/or emergency CS in PTB. Originally, it had been our intention to conduct three dedicated FGs with women from ethnic minority backgrounds [i.e. a group with women from Poland, a group with women of Pakistani ethnicity (Urdu/Punjabi speakers) and a group involving women of Somalian ethnicity], which we were intending to recruit from Bristol where a large Somalian ethnic population resides and one of our co-investigators (DS) was based when the original grant application was submitted. However, owing to Dimitrios Siassakos changing institutions to University College London (London, UK), a decision was made to substitute the group involving women of Somalian ethnicity with one comprising women of Indian ethnicity.
Recruitment: women
As recommended by Kitzinger,46 we aimed to recruit four to eight women to each group. In line with our original application, women were recruited via a variety of intermediaries and methods, and with the support of our partner organisations. For example, Bliss and Tommy’s used their established social media networks to advertise the study.
Our PG targeted women of Indian ethnicity who were able to communicate in English and had previous experience of PTB. Participants were recruited by a CASSAVA project research midwife based in a large tertiary maternity care setting in England. Potential participants were provided with written information (in English) about the study, including a participant information sheet and an opt-in form that was directly returnable to the qualitative research team. Women interested in participation could also contact the research midwife for further information. Five women agreed to participate, with three ultimately taking part (one woman changed her mind about participation prior to the event and one woman did not attend on the day).
Focus group 1 targeted women of Indian ethnicity who were able to communicate in English, with and without direct experience of PTB. Participants were recruited via our partner organisations, aided by a group member sharing the study information via social media (including school Facebook groups). Participants were asked to contact the qualitative research team for further information. Women were then sent an e-mail with the study information (in English) and invited to make contact for further information. Six women agreed to participate, with four women actually taking part (one woman changed her mind about participation prior to the event and one woman did not attend on the day).
Focus group 2 targeted women of Polish ethnicity with and without direct experience of PTB, including women who were contemplating pregnancy. It was organised by a bilingual Polish researcher (Ania Zubala) who recruited participants from actual and virtual Polish community groups, venues and forums. Basic information about the study, written in Polish, was displayed in hairdressers and shops attracting a Polish clientele and distributed via the internet and social media. Women interested in participating were advised to contact the bilingual researcher for further information. Nineteen women did so and were provided with a study information pack, written in Polish, with nine women returning the opt-in form. Nine women agreed to participate, with six women actually taking part (two women withdrew because of difficulties with childcare and one woman withdrew for an unspecified reason).
Recruitment into FGs 3–5 was under way when data collection was halted. One of these groups targeted women currently pregnant and attending a specialist clinic for women at high risk of PTB, another targeted women with previous experience of PTB and the final group was for women of Pakistani ethnicity (with recruitment via a bilingual Pakistani researcher). Fourteen women had indicated their agreement to take part in one of these FG discussions.
Data collection: women
The PG discussion was conducted on 21 November 2019 and FG 1 and FG 2 discussions were conducted on 4 February 2020 and 17 February 2020, respectively. All discussions took place in accessible and suitably private venues. At the start of each discussion, confidentiality was discussed and agreement reached on appropriate conduct. Women were asked to provide information, in confidence, about their reproductive history, ethnicity, religion, age and occupation. Some women chose not to share all aspects of this information and such decisions were respected. The PG and FG 1 discussions were conducted in English, whereas the FG 2 discussion was conducted mostly in Polish.
Focus group participants were presented with a hypothetical story (a vignette) and a series of questions, a method described by O’Dell et al. 47 The vignette (see Report Supplementary Material 8) described a situation in which a woman in spontaneous preterm labour was admitted to hospital, approached by a doctor and asked to consider taking part in the hypothetical trial. The vignette was designed to reflect a relatively common clinical scenario (i.e. spontaneous preterm labour at 30 weeks’ gestation). The vignette included an explanation of randomisation and covered important aspects of trial participation, such as the right to withdraw. Participants were asked to draw on their own experience and imagine how the woman in the vignette might respond to a series of questions (see Report Supplementary Material 9). These questions were designed to help the group explore and discuss trial participation in various clinical situations and at different gestations. The vignette and other FG materials were reviewed by a PPI group organised through one of the CASSAVA project’s partner organisations (Bliss) and also by one of the co-investigators, who checked the information for medical accuracy.
The discussion was facilitated by a qualitative researcher (SC, or, in the case of the Polish women’s FG, Ania Zubala), with the assistance of a colleague. Participants were encouraged to discuss the issues with each other, rather than with the facilitators. 48 Care was taken to ensure that all women had opportunities and felt able to contribute to the discussions. FG discussions averaged 90 minutes and were digitally recorded. Field notes were also taken.
Data analysis: focus groups with women
The first two transcripts were transcribed verbatim by a professional transcription company, whereas the transcript from FG 2 was translated and transcribed from Polish into English by its facilitator (Ania Zubala). In the light of the limited data we were able to collect, we decided to include data from the PG in our analysis. Consent to this had been obtained from PG participants at the start of their discussion. Our approach to analysis was similar to that described in relation to the health-care professional interviews (see Data collection), with close attention being given to how participants thought the character in the vignette ‘would’ or ‘should’ act and how they themselves would feel and act in a similar situation. We considered both the views of individual participants and the convergence and divergence at the group level. 49 We sought to identify both similarities and differences in women’s perspective(s) on being invited to take part in the proposed trial, including their impressions and concerns, and any changes that they thought might promote women’s participation. Particular attention was paid to potentially modifiable concerns relating to the conduct/delivery of the trial (e.g. informed consent processes). To enhance rigour, transcripts were read, data were coded and analytical reports were produced by two members of the team (SC and RIH). These reports were reviewed by, and discussed with, the wider qualitative team (JL and NH) before being used in the writing of this final report.
Results
We now report our key findings in relation to our four overlapping research aims. We focus principally on the findings from the interviews with health-care professionals because of the limited number of (women able to take part in) FGs. Some tentative insights from the FGs are reported at the end of the Results section.
Part 1: findings from interviews with health-care professionals
Achieved sample: health-care professional interviewees
Our final sample included health-care professionals with varying interests, levels of expertise and experience of working in more and less specialist sites. Further information is provided in Table 7.
| Characteristic | Number of health-care professionals |
|---|---|
| Obstetricians (N = 12) | |
| Consultant: obstetrics/fetal medicine | 5 |
| Consultant: obstetrics and gynaecology | 2 |
| Specialist trainee: obstetrics and gynaecology (all ST3–ST6) | 5 |
| Identifying/identified as a clinical academic | 8 |
| Experience of recruiting to clinical trials | 12 |
| Experience of PTB at < 28 weeks’ gestation | 12 |
| Current practice setting | |
| Tertiary care | 10 |
| Level 3 neonatal care | 10 |
| Level 2 neonatal care | 2 |
| Neonatologists (N = 5) | |
| Consultant: neonatology | 3 |
| Consultant: paediatrics (specialising in neonatology) | 2 |
| Identifying/identified as a clinical academic | 1 |
| Current practice setting | |
| Level 3 neonatal care | 5 |
| Midwives (N = 7) | |
| Midwife: clinical | 1 |
| Midwife: clinical/research | 1 |
| Research midwife | 4 |
| Senior research midwife | 1 |
Individual interviewees were given a unique reference, the initial letter(s) of which signifies their role [i.e. OB = obstetrician, N = neonatologist, (R)M = (Research) midwife]. Quoted interviewees are not further characterised in this report, as the size of the pool from which we were recruiting means such information could conceivably compromise participant anonymity.
Aim 1a: determine whether or not health-care professionals would be willing to recruit into the proposed trial
Health-care professionals would welcome improved evidence, but anticipate difficulties producing this
Health-care professionals largely welcomed the idea of a trial of MoB preterm (Box 4). Health-care professionals described the current evidence base for decision-making about MoB in PTB as suboptimal, with many highlighting gaps that they would like to see filled:
We really like guidelines and proformas and evidence-based work, and it doesn’t really feel like that is at the moment . . . it would be really good to have some really strong evidence to actually base our practice on, rather than just kind of [the] personal preference of the consultant.
M01
Health-care professionals largely considered the existing evidence base regarding MoB preterm as deficient, and consistently said that they would welcome improved evidence. However, many health-care professionals anticipated that delivery of the proposed trial would present significant challenges.
Health-care professionals described how the current deficiencies in evidence led to uncertainty (for both health-care professionals and pregnant women), difficulties facilitating informed decision-making and, potentially, suboptimal clinical outcomes for some women and babies. Therefore, interviewees were largely supportive of the idea of a trial:
We need really good evidence to drive change, so that a substantial number of women and babies can get better outcomes.
OB6
However, although health-care professionals were supportive, in principle, of a trial, interviewees envisaged significant challenges to delivering one as proposed in the HTP:
It’s a really good topic . . . it would be great to have the evidence . . . However, I think gathering the evidence is going to be difficult.
RM03
These challenges related mostly to the existence of strong and divergent views in the health-care professional community about the relative merits of vaginal birth and CS in PTB, and associated variations in practice (discussed in detail below). In addition, health-care professionals shared doubts about aspects of trial design, which they suggested might undermine wider support for the proposed trial and raised other, more pragmatic, concerns. These issues are explored in subsequent sections.
Health-care professionals report strong mode of birth preferences (i.e. areas where personal equipoise is lacking)
Despite, or because of, the gaps in the evidence base, interviewees said that they, and/or their colleagues, had developed quite strong and ‘fairly fixed’ (OB1) views about the best or safest MoB, and that views these guided their practice (Box 5). Interviewees also noted how these opinions and associated practices varied between, and sometimes within, centres:
Practice does . . . seem to be different in different centres. And quite strong, different attitudes in different centres, with not a very good evidence base to support it either way.
N02
Women could, theoretically, go down one route or the other if they just rocked up to a different hospital, and had a different person on shift.
OB6
Health-care professionals reported the existence of marked preferences for MoB in different clinical scenarios. These preferences appeared to emerge from a variety of sources, including unit/service norms, personal skills and confidence, direct experience, and memorable local and/or high-profile national cases. Although health-care professionals reported differing preferences and practices, some broad patterns were evident.
Factors influencing the development of mode of birth preferences
Reflecting on how these different MoB preferences had emerged, interviewees identified several overlapping influences. These included unit/service norms, personal skills and confidence, direct experience, and memorable local and/or high-profile national cases. The first of these influences (i.e. unit/service norms) was portrayed as playing a powerful role in shaping – sometimes by constraining – obstetricians’ (and others’) practices and preferences. Although some interviewees described how practices and preferences had developed through reflection and debate, others highlighted tradition and hierarchy as being pivotal influences:
It will be very much the culture of the hospital, how the deliveries are done . . . the way they’ve always done it.
OB9
Several obstetricians noted how senior consultants’ preferences regarding MoB effectively determined the training experiences of junior staff:
Trainees get trained up with what the consultants are comfortable with.
OB12
For instance, it was noted that where there was a strong tradition of delivering breech presentations by CS, encouragement to specialist trainees to consider vaginal birth, and opportunities to develop the necessary skills, could be very limited.
(Lack of) skills and confidence were further highlighted as important factors, and were implicated, in particular, in some individuals’ reluctance to undertake breech vaginal deliveries:
It’s meant to be evidence-based medicine, but you do . . . what you’re most comfortable with.
OB12
Doctors have become de-skilled in breech vaginal deliveries . . . and they don’t have the confidence or skills.
RM01
Personal experience was also said to play a powerful role in shaping many care decisions. Interviewees noted, for example, how difficult and/or distressing experiences could have a lasting impact on health-care professionals, and leave them keen to avoid repeats (prompting health-care professionals to make choices that supported that goal). Memorable (dramatic and usually traumatic) local cases and high-profile cases from other units/services (e.g. those receiving extensive media coverage because of neonatal deaths and/or disciplinary action against health-care professionals) appeared to exert a similar hold over less-experienced clinicians, as the following quotation from a specialist trainee illustrates:
I think that also, because of the Ninewells case50 – you know, with the consultant and the breech delivery, that was probably about 2 years ago – I think people are also a little bit more mindful, and sort of keen to go for a caesarean section, because . . . people would feel that there’s less likely a chance of sort of neonatal morbidity and mortality associated with a section – in the immediate aftermath at any rate . . . It [the Ninewells case] was a 24-week breech . . . a vaginal breech delivery was attempted . . . and essentially, unfortunately, baby died – and there was a decapitation.
OB4
Patterning of mode of birth preferences by clinical scenario
Health-care professionals explained that their decisions and practices were dynamic, contingent and responsive to a wide range of maternal, fetal and service/clinician factors. However, health-care professionals suggested that presentation (cephalic or breech) and gestational age were particularly prominent (and intersecting) considerations. Although interviewees articulated a variety of views, some broad patterns emerged, which are detailed below.
Health-care professionals suggested that cephalic presentations were generally considered lower risk than non-cephalic presentations. Therefore, a preference for attempting vaginal birth (and avoiding CS), although not unanimous, was relatively strong and widespread. In support of vaginal birth, interviewees cited avoidance of short- and long-term maternal morbidities potentially arising from CS; preservation of women’s reproductive capacity and future birth options; potential benefits to the foetus/baby, including enhanced respiratory and immune functions; and service considerations, such as meeting targets to reduce rates of CS.
For some interviewees, however, support for vaginal birth was contingent on gestational age. These health-care professionals reported that they and/or colleagues would be keenest to undertake vaginal birth – and feel most discomfort about undertaking CS – at later gestations:
30 weeks onwards . . . babies stand a better chance, they’ll have better outcomes generally . . . And if they’re a cephalic presentation, and normally grown, and a normal infant, then a normal vaginal delivery would be preferred.
RM02
The . . . later preterm ones, you know . . . if a baby was head down and it was 34 weeks, I would be quite reluctant to do a caesarean section . . . Personally . . . I’m not in equipoise at that . . . gestation.
OB8
For cephalic presentations at earlier gestations (i.e. less than 28–32 weeks), preferences for vaginal birth appeared less stable/strong, with several health-care professionals (including some obstetricians who reported their usual/default practice as vaginal birth) conceding to some uncertainty regarding what was actually the best/safest option:
The group that I agonise about are the sub-28 weekers, because those babies . . . are so teeny-tiny and they’re so fragile, that sometimes you think . . . ‘Does vaginal birth compromise them too much?’
OB8
Others, although themselves favouring vaginal birth, suggested that colleagues might take a different view, and explicitly advocate birth by CS at earlier gestations:
There’s a hardcore who already think that every preterm baby before ‘X’ weeks, 32 weeks, should be sectioned.
OB5
There was greatest consistency in perspective with regard to the most preterm cephalic foetuses (i.e. 22–25 weeks’ gestation). Here, vaginal birth was almost unanimously favoured, with many interviewees expressing profound unease about conducting CS in this scenario. Obstetricians, midwives and neonatologists all communicated concerns about the challenges of, and significant maternal morbidity associated with, the ‘classical’ section typically required:
Perform[ing] a caesarean at 22 weeks . . . is very difficult . . . [and] for future pregnancies it would be very different than a caesarean section performed later . . . I’m not sure someone would do a caesarean section at 22 weeks . . . [Possibly] from 24 [weeks], maybe 23-plus, but . . . [it’s] very borderline, because, you know, you could have a dead baby, so you just get an injury to the mum.
OB11
22-plus weeks, gosh, that’s really, well, less than the age of viability, isn’t it? . . . Why would you section a woman before 24 weeks? Why would you put a scar on her uterus, for a baby that was less than the age of viability?
RM02
Indeed, many obstetricians said, categorically, that they would not be prepared to conduct a CS at what they referred to as the ‘threshold of viability’.
With breech presentations, a preference for birth by CS was more common. Again, strength of feeling appeared to be affected by gestational age, with preferences for CS being most pronounced at later gestations (i.e. ≥ 28–32 weeks’ gestation). Accounting for this, health-care professionals highlighted a variety of concerns about the potential complications of a breech vaginal birth:
With the breeches, where they’re preterm . . . the lower half of the body can slip through a moderately dilated cervix, but . . . the cervix wouldn’t be large enough for the head to pass through . . . and then it’s an extremely stressful situation – when you’re at the point of no return and, you know, half of the baby is out, but the head gets stuck in the cervix, and then attempts have [to] be made to cut the cervix, with the baby’s head sort of millimetres away.
OB4
Owing to these risks, some interviewees said that they would never, or hardly ever, be happy to deliver a preterm breech baby vaginally:
A breech itself is risk, and a premature breech then is risk [added] to risk. So I would never be happy doing a vaginal breech on less than 37 weeks.
OB12
However, although recognising the challenges, around half the interviewed obstetricians said that they, and/or their colleagues, would at least consider vaginal birth in breech presentations when gestational age was < 28 weeks:
Many of my colleagues . . . are OK if it’s very early, [although] if they get to kind of 28 weeks and upwards, or a bit higher than that upwards, they would be less happy about a woman having a vaginal breech delivery.
OB1
As with cephalic presentations, obstetrician interviewees reported some uncertainty about the best way to manage breech presentations at these early (but not extreme) gestations.
Similarly (to cephalic presentations), at the very earliest gestations included in the trial, some interviewees suggested that they and/or colleagues might be reluctant to perform a CS:
With a breech delivery that was over . . . about 24 weeks, I would say they would do a caesarean section for that . . . [But] the little tiny ones, I don’t think they would . . . They obviously are thinking that they’re probably not going to survive anyway – so if they have a difficult breech delivery, it’s not going to make any difference.
RM03
Interviewees expressed a comparable reluctance to induce labour at these extremely early gestations. More generally, interviewees viewed elective birth scenarios as problematic in the context of the proposed trial. They explained that, in effect, this would involve comparing CS with induction. They considered this fundamentally different from a comparison of CS with spontaneous vaginal birth:
There is an important difference in babies whose birth you’re planning, versus babies whose birth isn’t planned . . . It feels to me that that’s a bit of a different question, because you’re factoring in [other] things . . . There are too many other variables, it’s not just, ‘Should this baby be born vaginally?’
OB8
Obstetricians also stressed that the decision to deliver electively, preterm, was never taken lightly, as the costs of premature birth were profound. Obstetricians explained that typically this would be contemplated only when a serious threat to maternal or fetal health had been identified. Several obstetricians emphasised that such situations tended to require urgent intervention and, as such, would lead them to favour birth by CS. These obstetricians indicated greatest willingness to consider induction at gestations close(st) to term.
Diverging from preferred mode of birth is more problematic in some scenarios than others
Health-care professionals recognised that, for the proposed trial to be successful, they and their colleagues would need to be willing to diverge from preferred MoB practices (Box 6). Although some health-care professionals suggested that, for them personally, commitment to evidence-based practice might act as something of a counterweight to imperfect personal equipoise, others expressed the view that getting people to move away from what they considered best/safest care would prove extremely challenging:
You’re going to be trying to get God knows how many DGHs [District General Hospitals] . . . to change what they see as safe care for the purposes of a research study. That’s not impossible, but . . .
OB6
Health-care professionals recognised that recruiting and randomising to the proposed trial might require diverging from MoB preferences. These health-care professionals suggested that the trial eligibility criteria include some scenarios/populations in which this shift was likely to be more difficult than in others. Interviewees noted that MoB preferences were at their strongest at the extremes of prematurity (i.e. closest to term and around the ‘threshold of viability’). In addition, health-care professionals often had marked MoB preferences for breech presentations and these tended to be strongest at gestations approaching term. In between the extremes (at roughly 26–32 weeks’ gestation) and in cephalic presentations, health-care professionals appeared to hold less firm and clear-cut preferences for MoB.
Trial eligible populations viewed as particularly problematic
Health-care professionals suggested that the trial eligibility criteria included some scenarios/populations in which convincing them and/or their colleagues to change their practice was likely to be more difficult than in others. They identified several populations/scenarios in which, they suggested, the risk of health-care professionals engaging in behaviours with clear potential to undermine the trial (discussed further below) was particularly high. These corresponded with those populations/scenarios in which MoB preferences were most marked (i.e. the extremes of gestation, breech births and elective birth), but also included some additional scenarios/groups (e.g. foetal compromise and women perceived as ‘vulnerable’).
For example, interviewees suggested that they and/or their colleagues would find randomising in situations when women (of any presentation) were very close to, or furthest from, term problematic:
I will randomise to any trial that people can convince me about equipoise. It’s just that . . . some of your inclusion/exclusion criteria are a little wide for me and I might not go as far . . . Would I randomise women at 36 weeks+6 days with a cephalic to caesarean section or vaginal delivery? In my heart, 100% not.
OB5
My suspicion is that some of the obstetricians, at the very lowest gestations, just won’t be willing to do a caesarean section and so might be unwilling to contemplate randomisation . . . I think some of them might have the view that the outcome is so poor for those infants, and the risks so large, that . . . it’s inappropriate to randomise.
N01
For some obstetricians, breech generally was considered problematic, because of the obstetricians’ strong preference for birth by CS:
The main sticking point for me is the breech thing . . . I wouldn’t really randomise [a] woman if knew she had . . . a premature breech . . . And I think a lot of other clinicians might feel the same.
OB12
Other scenarios in which some obstetricians questioned their own willingness to recruit and randomise included foetal compromise (e.g. growth-restricted babies):
It would depend if you could show me data that there was a potential benefit for having a vaginal delivery in these SGA [small for gestation age] IUGR [intrauterine growth restriction] babies . . . some data to show . . . there’s a definite benefit to having those contractions, to squeeze the fluid out of the lungs, because it means they’re less likely to have transient tachypnoea.
OB9
It was also suggested that clinicians might also be reluctant to recruit and randomise women they viewed as emotionally fragile or vulnerable. This might include:
Women who’ve had bad obstetric history, or are really anxious . . . and don’t want any sort of uncertainty. And prims as well . . . [Women having] first-time babies . . . are likely to be a lot more anxious . . . [Although] to be fair, everybody ought to have the opportunity to take part . . . so maybe we shouldn’t make assumptions . . . It’s difficult.’
RM04
Trial eligible populations viewed as less problematic
Conversely, interviewees suggested that where MoB preferences were less marked, they and/or their colleagues would find recruiting and randomising less problematic. These interviewees suggested that this might be the case between the extremes of prematurity (at roughly 26–32 weeks’ gestation). For example, one neonatologist remarked:
The 25, 26, 27-weekers . . . there is a very real question about how they should be delivered, whether it’s caesarean section or aiming for vaginal delivery. And . . . I think the obstetricians would find that an easier population to randomise.
N01
However, for some, this window of possibility was very tightly circumscribed in terms of gestational range and, for a few, presentation remained a prominent concern (with these interviewees suggesting that they would still only feel comfortable randomising cephalic presentations). As varying positions were expressed within interviews, pinning down the boundaries of personal equipoise often proved difficult:
For me, the biggest question is women who come in in spontaneous labour, and particularly the very preterm but viable infants. So more than 24 weeks – less than 26 . . . 28 weeks . . . 32 weeks would be definite cut-off.
OB08
The presence of such contradictions, corrections and/or qualifications in accounts suggest that, in many instances, interviewees were (re)formulating and (re)considering the certainty of their convictions as they talked. Explaining their hesitancies, interviewees highlighted the range of other factors that might, independently or in conjunction with gestational age and presentation, shift their preferences and practices around MoB. These included, inter alia, multiple pregnancies, degrees of fetal and maternal compromise, and other maternal characteristics, such as age and obstetric history. Interviewees stressed that these considerations were numerous, intersecting and could present in an almost infinite number of combinations. This, they said, made it ‘very difficult to know at exactly what point are you going to say, “I’m in equipoise”’ (OB5).
Marked mode of birth preferences are expected to lead to behaviours detrimental to the trial
Interviewees anticipated that the strong MoB preferences described above would prompt actions and behaviours at both unit/service and individual levels that would present tangible challenges to the proposed trial (Box 7).
Health-care professionals predicted that the existence of marked MoB preferences would induce behaviours that would potentially compromise the proposed trial. These behaviours included declining to open the trial, reluctance to recruit and randomise certain patients, directive counselling and/or deviation from protocol post randomisation.
Declining to host the trial
Interviewees emphasised that decisions to host a trial are made by teams rather than individuals and that their department would only sign up if there was unanimous support:
As a department [you] will have agreed to take part only if . . . you feel you can [all] support that conversation with women . . . We discuss our individual points of equipoise, and also where we feel there’s maybe not equipoise. And we’d only take it on if, as a group . . . we were all prepared to sign up for it.
N02
Echoing this suggestion, and based on conversations with obstetrician colleagues about the proposed trial, some research midwives (based in less research-active centres) expressed deep reservations that it would be possible to open the proposed trial at their centre:
We wouldn’t be able to agree to open this trial here because there would be no point . . . Equipoise is compromised . . . they’re saying that they’re not happy to do caesarean sections on someone that is not clinically indicated on.
RM03
Selectively recruiting patients
Health-care professionals often observed that, although they and/or their colleagues might make a general commitment to the trial, they/their colleagues might be reluctant (or even refuse) to recruit in those scenarios or populations in which practice preferences were strongest:
Something that you would have to allow for in the . . . protocol, is that, you know, some units may say, ‘Well, absolutely we’re not doing that at that gestation, but we’re prepared to do it at a lower gestation’, and vice versa. You may get some units that absolutely categorically refuse to section a 23-week woman, and others that will.
OB10
Health-care professionals suggested that this reluctance might also lead to more insidious and discreet selecting of patients clearly meeting trial eligibility criteria:
I think there’d be a lot of things like, ‘Oh well, maybe we shouldn’t include this person . . . I don’t think we should do a caesarean on them, so I don’t think we should randomise them’.
RM02
Counselling discouraging enrolment
Several obstetricians suggested that reservations about one MoB (either vaginal birth or CS) might also lead health-care professionals to counsel patients in less than impartial ways. These obstetricians warned that subtle modifications to recruitment conversations could significantly affect their outcome:
I would say . . . ‘We’ve got this trial . . . to see whether it’s safer to do caesarean or vaginal delivery . . . if you’re not on the trial, our general approach is to go for a normal [vaginal] delivery’. Now, If I don’t add that last bit, I . . . end up in an argument, ‘Well, you’re not going into the trial, but actually we’re not offering you [a] caesarean . . .’ So how you counsel people is never unbiased. Some people talk about non-directed counselling – it doesn’t exist!
OB5
Deviating from the protocol post randomisation
Finally, obstetricians warned of how discomfort with the practice(s) required by the protocol might result in deviations, in particular to health-care professionals defaulting to their preferred MoB post randomisation:
I think there will be protocol deviations. So, I think that if someone is randomised for section, they’ll get a section, no probs. I think if people are randomised to vaginal delivery, on that arm you’ll see more protocol deviations . . . documented as ‘Clinical reasons: decided caesarean section more safe’. Just because it’s so much easier.
OB4
Health-care professionals further noted how the above practices and deviations could have profound implications, including difficulties achieving recruitment targets and the final sample being skewed towards less problematic/controversial gestational ages and/or presentations. This, interviewees further warned, could result in findings that were open to challenge (with regard to their certainty and generalisability) and that could, therefore, ultimately be disregarded by health-care professionals who were disinclined to modify their preferred, habitual practices.
Aim 2a: explore if (and what) aspects of the trial design would need to change to make it more acceptable to health-care professionals
Modifying the trial protocol
Interviewees suggested that the chances of successfully delivering the trial would be increased by modifying the protocol, critically, by excluding those scenarios/populations in which MoB preferences were particularly marked (Box 8). Even those who personally viewed the proposed inclusion/exclusion criteria as ‘perfectly appropriate’ recognised that many colleagues would find them problematic. These individuals suggested that, as currently conceived, the trial would ‘bump into what is perceived reality on the shop floor’ (OB6). Different interviewees proposed excluding different scenarios/groups, including the extremes of prematurity, foetal compromise/anomaly, multiple pregnancies and elective deliveries. Excluding the first of these groups [i.e. babies furthest from and/or closest to term (both cephalic and breech)] received the most consistent attention:
I would bring up the lower limit gestation . . . bring the lower limit to 24 weeks . . . Including those 22, 23-week gestation babies . . . involves an area of pretty experimental medicine really. And then . . . presentation in the bigger babies . . . maybe bring your upper limit down to 34, rather than going all the way up to 36+6 – because I . . . accept that you’re going to put preterm breech babies in there, because that’s what you want to look at really, as much as the cephalic ones . . . but you’ve also got to balance that against the risk of the baby getting stuck.
N03
Some health-care professionals suggested that it might be necessary to change the trial design to make it widely acceptable. These health-care professionals noted that where MoB preferences were most marked, and support for the trial particularly weak/compromised, modifying trial inclusion/exclusion criteria might make it more acceptable. Interviewees emphasised that health-care professionals would also need to have full confidence in other aspects of trial design and suggested some further areas for attention and improvement. In addition, some health-care professionals felt that support for the trial might be improved by raising awareness of the (need for) evidence.
Ensuring that health-care professionals have confidence in the trial design
Interviewees emphasised that the trial needed to be highly robust in its design and scaled to ensure that it could deliver definitive answers. Reflecting on previous trials’ failures to deliver the clear-cut findings needed to bring about changes in practice, some interviewees suggested that pivotal design features, including the proposed (sub)sample sizes and outcome measures, warranted further thought.
(Sub)sample sizes
Although some interviewees welcomed the breadth/diversity of the study population, many questioned if – and what – subgroup analyses should be planned, and if sufficiently large subsamples would be achieved to support those analyses. These interviewees suggested that (perceived) weaknesses of this sort might undermine both health-care professionals’ support for (delivery of) the trial and, ultimately, their willingness to accept and act on its findings:
They’re going to have a real mixed bag of women, and different indications . . . it’s so diverse that as a practicing obstetrician, [whether] you would have enough confidence in its . . . power for these different groups . . . . I’m not sure.
OB01
I’m worried that you’re going to recruit such a . . . heterogeneous group . . . 20 of these, 40 of those, a 100 of those. And you won’t be able to do any subgroup analysis that’s of any meaning.
OB05
Outcome measures
Interviewees emphasised that the value of the trial hinged on collecting the right data on fetal/neonatal and maternal outcomes. The interviewees stressed that, for clear conclusions about the relative effects of the two MoB to be drawn, outcome measures needed to be capable of identifying relevant differences. Reflecting on the primary neonatal outcome (i.e. composite of alive at 6 months after birth or to home discharge, without significant IVH or cystic periventricular leukomalacia), some interviewees questioned if the frequency of these events was sufficient, especially at later gestations, to differentiate the outcomes of the two MoBs:
[In] that cohort of more-than-32-week infants, the chance of significant IVH is very small. [So] if the aim of caesarean delivery versus vaginal delivery is to avoid that, then it seems that the number needed . . . would be very high . . . that sort of outcome would be very difficult to study in a randomised trial.
N01
In addition, some neonatologists questioned if, and how, these measures were related to MoB:
I guess if . . . it [CS] was going to reduce the incidents of IVH or cystic periventricular leukomalacia, that would be . . . a very, very good outcome . . . [but] I’m struggling to think of a mechanism by which that would happen. Because in my experience, IVH, whilst you can get them fairly early on, often it’s not until after they’re born . . . And cystic PVL [periventricular leukomalacia] . . . [also] tends to be something that comes up later, and usually there’s been something to explain it . . . some kind of post-delivery insult . . . I’ve never really had a feeling that . . . they’re related to the way they were born.
N04
Many interviewees emphasised that what was important to parents, perhaps even more so than clinicians, was how babies/children developed over subsequent months and years. These interviewees noted that measures such as IVH and cystic periventricular leukomalacia were essentially proxies for such outcomes:
As neonatologists we are often thinking that what matters most is the outcome of the child in later childhood, and conscious that when you look at surrogate markers like IVH, although it’s clearly important, that occasionally the later outcome doesn’t agree . . . If this were designed as a neonatal trial, rather than an obstetric trial, I suspect the primary outcome would be neurodevelopment, rather than survival or IVH.
N01
These interviewees said that, ideally, these longer-term developmental outcomes should be measured directly. A few (obstetricians) suggested that capturing longer-term maternal outcomes was important, too:
If your primary outcome measure is fetal handicap [and] survival . . . that’s fine . . . [but] the problem is the longer-term outcome. So like the problems of increasing caesarean section rates . . . 15–20 years [on] people get menstrual disturbance . . . there’s a much higher rate of placenta praevia and accreta, but that’s a consequence in 3, 5, 10 years’ time – studies don’t look at that.
OB05
However, this lack of longer-term outcome measures was also acknowledged as being a weakness common to many (obstetrics) trials, and reflective of the restricted nature of much research funding.
Raising awareness about the (need for) evidence
Although our interviewees portrayed themselves as cognisant of the need for evidence, some questioned whether or not health-care professionals more widely were aware of the limitations of the evidence, with one midwife, for example, describing her colleagues as ‘very shocked’ to hear that there was minimal evidence to support current practice(s). Therefore, some health-care professionals surmised that a starting point in securing health-care professionals’ support for the trial would have to be raising awareness of the current state of the evidence base. Obstetricians emphasised that those clinical scenarios in which MoB preferences were especially marked (e.g. at the extremes of gestation) would need particular, focused attention. Theses obstetricians suggested that, in so doing, it would be critical to have credible advocates/champions making the case for the trial:
. . . a prominent face . . . a big name.
OB4
Others emphasised the importance of identifying and getting the support of local decision-makers and opinion leaders:
People will follow the lead . . . follow the local academic lead . . . So long as it’s not perceived as blatantly unsafe, then they will follow that lead and randomise.
OB10
Aim 3: determine if there are any practical, logistical or other challenges likely to impede trial delivery, and establish what resourcing and other support health-care professionals might need to overcome these
Concerns about the sufficiency of clinical and research resources
Interviewees drew attention to workload issues that might impair their own, or their colleagues’ at other (smaller) centres, capacity to contribute to the trial (Box 9):
It’s time, isn’t it? It’s like ‘busy-busy’!
RM03
Interviewees also highlighted more pragmatic issues that might make trial delivery challenging. These related, principally, to (1) service and research resources and (2) health-care professionals’ clinical and research skills. Interviewees suggested that – in some units/services – significant support (investment and training) would be needed to make a future trial feasible.
Interviewees emphasised that, as well as having an impact on the workload of obstetricians, midwives and labour ward staff, MoB decisions had implications for anaesthetists’ time, theatre usage and labour ward bed occupancy. Interviewees noted how, with more work to do than time to do it in, health-care professionals might, quite reasonably, prioritise other tasks:
A challenge with any clinical trial, [is] getting people to take part in research when they’re already very busy with their clinical jobs . . . although everybody’s aware of research, you know, it can sometimes just be seen as a bit of a nuisance on top of your . . . busy NHS job.
OB3
Many (obstetricians) expressed the view that recruitment would need to be ‘clinician led’, as discussions about MoB require clinician expertise. However, these interviewees also acknowledged that they would need the help of research support staff, including Good Clinical Practice (GCP)-trained research midwives, in identifying, screening and randomising eligible patients:
Even if the clinician took consent, they’d often need a little bit of support with the randomisation process, and that can be not just inexperience of doing it, but also just workload. And, therefore, when you’ve got . . . research support staff . . . you get better recruitment.
OB7
Interviewees noted how patients could present 24/7, with some commenting that research midwives worked largely office hours. Therefore, even at those sites with more developed research infrastructure and support, interviewees envisaged challenges recruiting and randomising out of hours. It was noted that a large group of (clinical) staff would need to be willing and able to provide support to the study if eligible patients were not to be missed. As interviewees further noted, these people would need GCP certification and also, some suggested, some trial-specific training, as well as a proportionate amount of ring-fenced time:
Centres that become involved in a study like this have to really commit to it, and have a number of individuals who are very dedicated to it . . . And you probably need to have a certain amount of dedicated resource. I don’t think it’s enough . . . to simply expect people to take it on as part of their day-to-day workload.
N05
Concerns about the adequacy of (some) health-care professionals’ clinical skills
Interviewees questioned how many units/services could guarantee the availability of staff with the necessary skills to deliver both arms of the proposed trial effectively. Obstetricians emphasised the criticality of this issue in the more challenging birth scenarios encompassed by the trial (e.g. breech vaginal birth or very early CS):
These extreme preterm gestations, you can’t just leave it to the registrar, the junior registrar, to do these deliveries. These are deliveries that need input from experienced people.
OB9
Several interviewees, although emphasising that their own (large, tertiary) service had 24-hour consultant cover [‘There’s always two obstetricians on-call’ (OB11)], warned that this was not the case at other centres. Interviewees surmised that the availability of staff with appropriate clinical skills and confidence would have implications for both recruitment and study outcomes. Therefore, some interviewees suggested that the trial team should focus on tertiary units where there was greater experience of both difficult vaginal births and CSs and 24-hour consultant cover:
My unit has 24 hours – they can sort of cover – so it makes no difference really what time of the day or night it happens . . . that wouldn’t affect it. But I guess if you’re trying to randomise in other units it would, and that’s potentially a problem . . . I think you probably wouldn’t want to do this . . . in units where there isn’t experienced obstetric cover available for breech vaginal delivery – because that’s necessarily going to bias your results.
OB10
Although some interviewees surmised that in the bigger clinical units appropriate skills and confidence could be assumed, others suggested that for some clinical scenarios, in particular breech vaginal birth, even ‘experienced’ clinicians might vary in their skills and confidence. Health-care professionals drew attention to a general shift away from vaginal breech birth following the completion of the Term Breech trial. 13 Some interviewees remarked that, as a consequence of that trial, there had been limited/diminishing opportunities for junior doctors to acquire and embed the necessary skills and confidence to deliver babies this way. Although many interviewees saw this as a major stumbling block for the trial, some suggested that investment in skill development and support might increase the pool of health-care professionals (i.e. obstetricians) with the skills and confidence necessary to undertake these more complex vaginal births successfully. Proposals included offering ‘a refresher course about vaginal breech’ (OB4) and ensuring that less-experienced obstetricians had ‘some senior support’ (OB12) when undertaking this MoB.
Some interviewees additionally emphasised that birth by CS could also be difficult. These interviewees noted, in particular, that very early CS, especially ‘classical caesareans’ involving a long vertical incision, presented significant technical challenges, again, warranting the involvement of a senior, experienced obstetrician. Some interviewees opined that the profession lacked experience and the necessary technical expertise to successfully undertake CS at the earliest gestations covered by the trial (i.e. 22–24 weeks’ gestation):
I’d expect a caesarean section to be more difficult [at 22–24 weeks], so there is a technical issue here. We’re not really expert in doing a caesarean at these gestational ages.
OB11
Concerns about the adequacy of (some) health-care professionals’ research skills
Alongside identifying gaps in clinical skills, interviewees questioned whether or not they and/or colleagues had the necessary research skills. In particular, concerns related to the challenges of securing informed consent intrapartum. Health-care professionals expected these conversations to be complex and challenging, especially when PTB was entirely unexpected:
Women in high-risk groups . . . they’re an easy-ish group to speak to because they have prior knowledge and prior experience . . . and they’re out with the kind of labour scenario. Now the challenge is . . . a lot of the women that come in in preterm labour are just coming in in preterm labour . . . Speaking to somebody who’s in labour is harder.
OB1
Many interviewees expressed personal discomfort about broaching trial participation with women who were in active preterm labour. Others interviewees said that they expected colleagues to have concerns and some anticipated significant reluctance to be involved:
No-one will want to do the study, with the exception of weirdos like me! Every single R&I [Research & Innovation] midwife and nurse is going to try to not consent women for us . . . because it’s an uncomfortable conversation.
OB6
Intrapartum recruitment (to any trial) was perceived as presenting distinct practical and ethical challenges. Several health-care professionals expressed uncertainty about women’s capacity for decision-making in the light of pain, analgesia and – in the context of PTB – (di)stress. Some health-care professionals questioned if, under these conditions, informed consent was achievable:
A caesarean in one pregnancy . . . restricts your options for the next pregnancy. But that’s a difficult decision for women to make when they’re not in labour and they’re not worried about having a preterm baby . . . Asking them to do that when they’ve got all that going on . . . I would not be entirely happy . . .. I think it’ll be very difficult for a woman and her partner to give truly informed consent under those circumstances.
OB1
The need to ensure that women were aware of the potentially serious and enduring consequences of the two MoBs was viewed as compounding the difficulty of the conversations that health-care professionals needed to have:
That would be a really difficult thing to consent women for . . . it would be a massive conversation.
M01
Health-care professionals’ comfort with the idea of seeking informed consent intrapartum appeared to be related to the extent of their prior experience of intra- and peripartum trials. Those health-care professionals with more experience reported feeling more comfortable with the idea. A few interviewees suggested that training and tools might help less experienced health-care professionals feel more at ease undertaking this work. Some health-care professionals went further, arguing that training around consent would be essential:
[Clinicians] need a lot of prep[aration] about the consent process . . . a lot of support through that discussion, and a lot of case scenarios, and a lot of working out how you can take informed consent in different situations in an intrapartum setting.
OB7
Refining the consent process
Health-care professionals also proposed some refinements to the trial consent process. Many health-care professionals were in favour of introducing the trial to women earlier in their pregnancy (i.e. before they went into labour) and some opined that this could provide a basis for – and, indeed, should be a condition of – the securing of verbal consent intrapartum:
Having an option for verbal consent is a good idea . . . having the written consent afterwards . . . [But] I think you’d have to have quite a good way of informing women about the trial [ahead of time] . . . Because with verbal consent, I don’t think the . . . time they can give verbal consent is the first time they’ve ever heard about the trial, within the consent guidelines that we’ve worked within.
OB07
Several health-care professionals suggested making all women aware of the trial by mentioning it and/or distributing leaflets at key appointments (e.g. at the 20-week scan). Some health-care professionals noted that an alternative, more targeted, approach would be to make women identified as at risk of PTB, who were often seen in specialist clinics, aware of the trial at their appointments. However, interviewees noted that publicising the study – even in the latter, more targeted way – would result in many women who ultimately gave birth at term receiving information. This, they said, could have costs for both health-care professionals and patients, creating work for the former and, potentially, unnecessary anxiety for the latter:
If you introduce the possibility of preterm labour early on in pregnancy, you’re . . . potentially worrying, or raising concerns in, a lot of women who will not go on to deliver preterm.
N05
Aim 4: understand and explore the potentially distinctive challenges of recruiting ethnic minority women into the trial, and establish any additional resourcing and support needed to optimise these women’s participation
The complex trial design we were tasked with unpicking with health-care professionals left limited time in interviews to explore their perspectives on the challenges of recruiting ethnic minority women. Consequently, we captured only a small number of data on this topic.
Health-care professionals emphasised the diversity of the populations they served and described anticipating particular difficulties recruiting ethnic minority women into the proposed trial:
My personal . . . experience . . . was that it’s . . . much more difficult to recruit, you know, all ethnicities that are not white European to medical trials . . . so even similar, sort of British-born but ethnically not white people . . . Patients are often extremely well motivated, and very altruistic . . . but my personal finding has been that it’s more difficult to get consent from non-whites, non-white European ethnic groups.
OB4
Different cultural frameworks and expectations (regarding health care, birth/labour and research) were suggested as potentially affecting women’s responses to trial invitations:
Also, yeah, cultural – cultural implications would be really interesting. So, for example, lots of women who have come from kind of Africa, they kind of just get on with it . . . just keep going – ‘What will be will be’. And obviously you’ve got all the family influence . . . the cultural influence, you know, the religious influence . . . all of that is definitely relevant . . . and I think would affect people’s opinions of being part of the trial.
M01
Profound difficulties were anticipated when women did not speak English as their first language, and the support of interpreters was required. Challenges identified by health-care professionals included getting hold of a suitable interpreter when the need for one had not been anticipated and ensuring the integrity of information relayed through a third party:
It’s an extra barrier . . . if they don’t speak English they certainly won’t read English . . . [and] they may not even be able to read their language either . . . if we’re approaching them in clinic, or something that’s been booked, then an interpreter is usually there, but it’s a bit more difficult if they turn up unannounced.
RM04
We’d have to use interpreters, which would be interesting as well. So that adds another level of bias . . . I’m sure they [interpreters] will have an opinion about it.
M01
These potential language barriers compounded health-care professionals’ anxieties about the feasibility of securing informed consent. Similar concerns were expressed by women taking part in the FGs (see Part 2: findings from the focus groups with women).
Part 2: findings from the focus groups with women
Although (as discussed previously) we did not achieve data saturation in this element of our study, some issues were prominent in the discussions. These issues warrant reporting, as they indicate potentially important concerns about trial participation.
Achieved sample: pilot group/focus group participants
The characteristics of our achieved sample of women are detailed in Table 8. Individual/quoted pilot and FG participants are not characterised, as this sort of information might compromise their anonymity.
| Characteristic | Number of participants |
|---|---|
| Age (years), median (range) | 35 (24–43) |
| Ethnicity | |
| British Indian/Indian | 6 |
| Polish | 6 |
| Not disclosed | 1 |
| Religion | |
| Hindu | 2 |
| Muslim | 1 |
| Sikh | 2 |
| Christian (Catholic) | 3 |
| Not disclosed | 5 |
| Occupation | |
| Professional | 7 |
| Semi-professional/skilled | 2 |
| Unskilled | 1 |
| Student | 1 |
| Full-time carer | 2 |
| Reproductive history | |
| Has children | 10 |
| Previous vaginal birth | 7 |
| Previous CS | 5 |
| Previous PTB | 7 |
| Previous miscarriage/loss | 6 |
Aim 1b: determining whether or not women would be willing to participate in the trial
‘A big decision’ with risk of allocation to unnecessary surgery
Focus group participants highlighted the significant and very different short- and long-term implications of the two MoBs investigated in the trial. In particular, participants raised concerns about the potential for trial participation/randomisation to result in a woman having unnecessary and invasive surgery. Participants also expressed scepticism about women’s willingness to accept randomisation to CS, in the absence of a decisive medical indication for such a procedure (Box 10):
I would be worried that they would assign me to a C-section, although there might not be health indications towards this. I would prefer to give birth naturally.
FG 2/P3
Why would you opt for major surgery, unless you really had to have it?
FG 1/P2
Across the groups, participants highlighted some issues and concerns that might potentially affect women’s willingness to take part in the proposed trial. These concerns are encapsulated in the following quotations/phrases: ‘a big decision’ (FG 1/P4), a bad time and ‘the doctor (still) has their part to play’ (FG 2/P1).
These women highlighted the surgical risks of CS (e.g. bleeding and infection), lengthy recovery times, impact on one’s ability to provide care for the new baby (and any other children) and implications for future pregnancies/births. These women noted how these possibilities might lead them to favour a vaginal birth:
I might have an inclination to want to have a vaginal birth, just because if you have a C-section, you’re more likely to have to have a C-section the second time round.
PG/P2
Having had a C-section, if I could have had a normal delivery, normal vaginal delivery . . . I think I would have taken that over the C-section any day, because I wouldn’t have had a major operation, I wouldn’t have had 6-plus weeks of recovery, you know?
FG 1/P3
However, as the second of the two quotations above suggests, views on the desirability or otherwise of the two MoBs were shaped by women’s prior experiences. Indeed, those who had experienced complications during, or because of, vaginal birth emphasised that this MoB too could have serious and burdensome implications:
I had a third-degree tear and other issues, so literally as soon as I had him, I was off in surgery anyway . . . and that was with a vaginal birth.
FG 1/P1
I’ve had both a natural birth and a C-section and I’m not really sure what is worse – as I had forceps delivery, which was not fun at all, I had a cut, and they stitched it badly.
FG 2/P4
Women without prior experience of childbirth appeared not to have (such marked) MoB preferences. Nevertheless, they still viewed the decision as a significant one.
A bad time: discomfort with intrapartum consent
Women in all groups found the idea of being approached and consented in the intrapartum period deeply problematic:
If she’s in labour, obviously her waters have broken, she’s panicked and she’s being told to make a decision. . . It’s quite a big decision . . . to be introduced to it at that point!
FG 1/P4
It’s . . . about timing, and being able to think straight . . . you have to be in the right frame of mind to make important decisions.
PG/P2
Those who had experienced a spontaneous PTB highlighted how ill-prepared they had been for the premature onset of labour. Moreover, FG participants who had previously given birth emphasised how labour more generally affected women’s ability to concentrate, highlighting the effects of shock, pain, medication and a narrowed focus on ensuring optimal foetal outcomes:
During labour a person stops thinking and focuses only on this eventually ending, on the child being healthy, on themselves being well . . . This is what I think every mother thinks during labour.
FG 2/P3
Having ‘so many more important things going through our minds’ (PG/P2) might, as FG participants suggested, make women unreceptive to approaches about trial participation:
It is just the last thing on your mind, it’s absolutely the last thing.
FG 1/P3
Here I am, about to give birth, I’m in pain all over, I’m worried . . . and someone approaches me and asks about some research study. First thought? ‘Damn, just piss off! I don’t give a damn about your research just now, I just want to make sure that all is well with my child’.
FG 2/P4
Other women noted the potential challenges of securing genuinely informed consent. To illustrate this point, a FG participant who had previously had an emergency CS questioned if the consent she had given could really be considered informed:
I was asked to sign loads of consent forms and I don’t even have a clue what half of them were, because someone was just telling me, ‘You need to sign this in order to get the babies out, to give them a chance to live’, and I’m like, ‘Fine – I’ll sign whatever it takes!’
PG/P2
In the light of these concerns, women in all groups favoured introducing the trial earlier in pregnancy (somewhere between 9 and 20 weeks):
If someone had mentioned something beforehand, so you were aware . . . [of] this possibility.
FG 1/P1
I think planting a seed earlier on, giving some literature or a leaflet to say, ‘Look, this is a study that’s happening. It’s not to say that you’re going to have a preterm birth, but it’s an interesting read. Have a think about it’.
PG/P1
Reflecting further, some women suggested inviting women to indicate interest in trial participation in their birth plans:
She could include this in her birth planner, whether she would like to agree to such a thing . . . It’s obvious that things do not go according to plan every time, but . . . midwives always try to consider the birth plan the woman has with her.
FG 2/P2
Although some participants drew attention to the potential of early information to induce unnecessary anxiety in women who would ultimately deliver at term, others felt that, if information was composed and provided in careful and sensitive ways, this could be avoided. Moreover, some participants were strongly in favour of raising awareness of, and preparing women for, the possibility of PTB, and surmised that trial information might help achieve this:
That would be good, to raise awareness among those who could potentially – just generally, among all mothers, as you don’t know who . . . might have a preterm birth – that there is such an option, and that something like this might happen . . . [Then] they are able to prepare themselves for this emotionally.
FG 2/P2
‘The doctor (still) has their part to play’: relying on health-care professionals to safeguard women’s interests
Focus group participants emphasised that health-care professionals remained an important part of the equation and women’s agreement to enrol in the potential trial did not – or should not – relieve health-care professionals of responsibility to consider and safeguard their interests. To the contrary, women expected health-care professionals to assess individual women’s suitability very carefully and consider whether or not, in the light of factors specific to that individual, they could truly claim to be in equipoise:
I think it’s important that the doctor is considering what the safest option is, and you’re only having the random choice made by the computer as long as there’s nothing else . . . no other factor that would be important to consider.
PG/P2
Participants highlighted how the clinical (and social) circumstances of PTB were highly variable and stressed that women wanted and expected to be treated as individuals, and counselled accordingly. Although participants did not necessarily see this a barrier to trial participation, they stressed that the information given to women about trial participation and the pros and cons of the two MoBs should be tailored to their particular circumstances. For example, FG 2 participants suggested that women’s perspectives and concerns might be quite different across the gestational age range covered by the trial and PG members suggested that health-care professionals should give women detailed information about the respective risks and benefits of the two MoBs at the gestation they were at. The PG members noted, for example, that there were different ways of performing a CS and that the type of incision made would be influenced by individual factors, including the size of the uterus and whether it was a singleton or twin pregnancy:
They could . . . say, ‘Well, these are the risks associated with us doing an incision this way, as opposed to that way, because the baby is extremely premature’.
PG/P1
Health-care professionals also had views on women’s willingness to participate, as described in Box 11.
In the light of the limitations of our FG data, we also report the perspectives of interviewed health-care professionals. These were quite variable. Some health-care professionals, for example, were optimistic, and suggested that trial enrolment might offer women a rational way of dealing with uncertainty and the difficulties for decision-making that creates. A few health-care professionals noted how the prospect and/or experience of risk, and loss, could act as incentives for women to support research:
If women realise that they’re high risk for something, often then women are keen to do trials and get a better understanding of what could be the best care.
OB12
However, others, in particular midwife interviewees, offered a more sceptical view, and noted how some women – like themselves – had strong MoB preferences that might act as barriers to recruitment. These interviewees also surmised that women might be resistant to having their care determined through a randomisation process and, instead, look to the care team for more individualised care:
I don’t think it will be positively received by women, because I think they’ll want to know from us which is the safest option, they won’t want to be randomised.
M02
Where health-care professional interviewees were very much in agreement was that women wanted ‘what was best for the baby’ (RM01) and would prioritise their unborn child’s well-being over both risks to themselves and potential benefits to others. It was noted that women would, therefore, need strong reassurance that the risks to the baby of the two MoBs were comparable:
Everybody’s an individual . . . [with] different experiences and pasts . . . there may be some women who particularly want to do things one way or another, but I think the majority of women, their number one priority . . . will be the safety of their baby, whatever is best for them . . . [So] in terms of the study, you’d have to be able to reassure them that neither one way nor the other would increase the risk for the baby . . . you’re just back to the usual risks and benefits of section, essentially . . . And [that] will just vary from case to case as well, depending on the reason for needing delivery.
RM04
Aim 4: to understand and explore the potentially distinctive challenges of recruiting ethnic minority women into the trial, and establish any additional resourcing and support needed to optimise these women’s participation
Family and autonomy
Women from the three groups reflected on ownership of, and influences over, decisions about MoB and participation in the hypothetical trial. Women in the PG and in FG 1 (all of Indian ethnicity) mostly framed the decision as one they would make jointly with their husband/partner, with some questioning whether or not women could make such a decision independently. The potential for women and their husbands/partners to have different priorities, and for different opinions, therefore, to emerge, was noted by members of FG 1 (Box 12). One woman questioned what might happen should there be a marked difference of opinion between a woman and her husband/partner:
There could be a conflict of what happens, because there’s an emotional side and a physical side . . . If it’s a conflict, then what happens in that situation? Because the baby’s joint. Just because the woman’s delivering it, the responsibility is on both.
FG 1/P4
The three groups highlighted different potential challenges to recruiting ethnic minority women: family and autonomy (discussed by the PG, FG 1 and more fleetingly by FG 2), language and consent (discussed by FG 1) and expectations of health care (discussed by FG 2). Women across the groups highlighted the difficulties/limitations of generalising about the experiences, outlook and needs of women who might have a shared/common heritage, but very diverse experiences and circumstances.
Another FG 1 participant commented that every relationship was different, and warned that not all were supportive/healthy. Therefore, she surmised, discussions about MoB and trial participation should, in the first instance, ‘be had privately with [women]. I mean, ultimately there’s a confidentiality issue’ (FG 1/P3).
Participants in the PG and in FG 1 further noted how the lives of women of Indian heritage might be closely entwined with those of their extended families. Some participants explained that they lived with their husband’s family (e.g. their mother- and father-in-law). Those who lived independently observed that their ‘almost westernised’ living arrangements were perhaps ‘not the usual Indian thing’ (PG/P1).
Women in these two groups noted that extended family could be an important source of support, but also (potentially) a cause of unhelpful interference and stress. In the case of decision-making about trial participation, some women surmised that older family members would be against this and, given the opportunity, might express their views strongly:
I live with my in-laws. They’re quite old-fashioned, so anything like this, they’d be like, ‘No, you’re not going to – don’t take part!’
FG 1/P3
However, others remarked that, in their case, these family members were/had been medical professionals and might, therefore, be quite supportive. Some PG participants suggested that although the perspectives of people other than their husband/partner might have little influence on the decision they made, the airing of opinions might nevertheless make that decision harder to live with. For this (and other reasons), these women said that they might limit the information they shared with their extended family.
Language and consent
Focus group 1 considered the issue of language and the implications of this for consent. Although all group members were professionals who spoke English as their first language, they noted that language might present a barrier to participation for some other women with Indian backgrounds. These group members warned that these women might be unclear as to what they were being asked to do, but feel ‘embarrassed to say that [they] don’t understand’ (FG 1/P2). This lack of understanding, they suggested, might discourage participation and lead to a less diverse sample than the triallists had hoped for. In addition, the group members emphasised that unresolved language barriers could have significant implications for the quality of women’s consent and raised serious doubts as to whether or not consent could, in such situations, be considered ‘informed’. Therefore, the group members suggested that, when English was not a woman’s first language, the involvement of an interpreter was absolutely key:
You need to have a translator explain everything, even if you think they understand, . . . because . . . it’s a big decision and . . . it’s important that they are actually fully informed.
FG 1/P2
Expectations of health care
Women in FG 2, all of whom were first-generation arrivals from Poland, reflected at length on the differences between reproductive and maternity health care in Poland and the UK. Theses women’s accounts suggested that experience of other health-care systems might create different expectations of UK health-care professionals and health care, as well as affecting perceptions of the desirability, or otherwise, of specific medical procedures, with these issues potentially having implications for decisions about participation in an intrapartum trial.
These women emphasised how pregnant women in Poland were monitored very closely, from conception onwards, and contrasted this approach with practices in Scotland/the UK:
[Here] until the 12th week, the pregnancy is not considered a pregnancy . . . they simply don’t support pregnancies prior to the 12th week . . . [whereas] in Poland they do everything to fight for these babies.
FG 2/P2
Another thing I’ve noticed here . . . is the attitude towards pregnant women . . . In Poland you have ultrasound scans done very frequently . . . they test your glucose levels . . . there’s loads of checks.
FG 2/P3
Women suggested that (what they perceived as) the more hands-off approach that is usual in Scotland/the UK could create unease among a community of women accustomed to, and/or expectant of, closer and more attentive care. Some women reported that this had prompted them to consult a Polish health-care professional privately, with one woman warning others that this had, however, led to further worries (because of the conflicting medical opinions that emerged on the appropriate management of their pregnancy).
Women noted how differences in care/management practices continued up to and, indeed, beyond birth, with several group members suggesting that there was a reluctance to admit pregnant women to hospital in Scotland/the UK until labour was quite advanced. Once admitted, some women felt that their choices were limited, with one woman asserting that ‘[i]n this country . . . there is an extreme pressure to give birth naturally’ (FG 2/P2). Women suggested that this included scenarios in which (in Poland) a CS would be considered the more appropriate option. For example, women noted how assisted/instrumental deliveries considered outdated and, indeed, ‘dangerous’ in Poland remained relatively common in the UK:
In Poland . . . forceps are not used any more . . . this practice has stopped many years ago.
FG 2/P4
With regard to how these (unmet) expectations of care and perceptions of good management/birth practices might affect decisions about trial participation, women suggested that they might fuel an innate (national) anxiety:
In my opinion we, Polish women, are anxious.
FG 2/P3
We are anxious, yes.
FG 2/P2
Women further surmised that trial participation might conflict with Polish women’s need to feel ‘in control of everything’ (FG 2/P3). Moreover, these women’s expectations of close and tailored care would seem incongruent with the protocol-driven care inherent in a trial and, as such, might plausibly discourage/disincentivise trial enrolment further.
Discussion
Health-care professionals described the existing evidence base for MoB in PTB as deficient and said that they would like stronger research evidence to inform future clinical practice. Therefore, health-care professionals described largely welcoming the idea of the proposed RCT. However, health-care professionals also anticipated significant challenges to delivering the proposed trial. They explained that – despite the appearance of clinical equipoise – as individuals they, and/or their colleagues, often had quite marked and varied MoB preferences. These differences appeared to arise from a variety of factors, including unit/service norms, personal skills and confidence, direct experience and memorable local and/or high-profile national cases. Preferences were particularly strong in relation to very late and very early (preterm) gestations, as well as breech presentations. Health-care professionals surmised that such preferences would severely affect their and/or their colleagues’ willingness to recruit and randomise particular groups of women into the proposed trial. Health-care professionals suggested that awareness-raising activities and, more crucially, protocol modification might increase health-care professionals’ willingness to recruit. With regard to the latter, the need to tighten the inclusion criteria and, ideally, review outcome measures was widely highlighted and discussed. Even with these modifications, other more pragmatic concerns [relating to the (in)adequacy of clinical and research resources and development of appropriate clinical and research skills] were perceived as likely to make trial delivery challenging. It was suggested that significant investment would be needed – in both staff training and broader resourcing and support (e.g. ring-fenced time) – to resolve those concerns. It was also noted how, to promote the inclusion of ethnic minority women, suitable language provisioning would be necessary.
So how do these findings add to what was known already? As noted earlier, previous efforts to conduct RCTs of MoB in PTB have been unsuccessful, with health-care professionals’ reluctance to recruit/randomise being suggested as a pivotal factor. 3 Our research indicates that this is a plausible assertion and, moreover, it is one that is likely to present ongoing challenges for triallists keen to resolve the clinical uncertainties around MoB in PTB, especially if the design outlined in the HTP is used. Critically, our data offers insights into why, when and to what extent health-care professionals might be reluctant to recruit. These insights can offer useful guidance moving forward. Specifically, these insights can help inform decision-making about which inclusion criteria should be retained, and which modified, to make a trial a more feasible option.
In addition, our enquiries prompted health-care professionals to propose (other) strategies that might (possibly) encourage and enable more health-care professionals to support and recruit women into a future trial. These included awareness-raising activities and supporting health-care professionals to develop appropriate clinical and research-related skills. Clearly, some of these strategies would be easier and less costly to implement than others. For example, using research champions to raise awareness is a relatively low-cost option, whereas training/upskilling health-care professionals to perform complex vaginal births and very early CSs with confidence would be considerably harder, more time-consuming and expensive. If a trial were to go ahead in the future, the effectiveness of such interventions in promoting health-care professionals’ engagement might usefully be evaluated in an (inbuilt) pilot phase.
With regard to women’s willingness to participate, our understanding of this issue is best described as incomplete. Data from health-care professional interviewees suggest that women, like themselves, may have strong MoB preferences and that these might affect their willingness to take part in a future trial and accept randomisation. There was some support for these assertions in the FG data. The data available from the FG also indicates two additional concerns that warrant careful consideration. The first concern centres on women’s anxieties about undergoing a (potentially) medically unnecessary and invasive procedure (i.e. a CS) as a result of trial participation. The second concern, which was also shared by some health-care professionals, involves the challenges and ethics of asking women to consent to trial participation during labour. This finding is not altogether surprising, as qualitative work undertaken during a peripartum trial involving women who had a retained placenta likewise highlighted the challenges of obtaining/giving informed consent in situations in which women are anxious, distracted and experiencing the effects of analgesics. 51 Indeed, like our FG participants, the women who took part in the retained placenta trial could see clear benefits to trial information being cascaded during the antenatal period and saw these as outweighing the potential risks/costs of (unnecessary) anxiety and distress. 51 Moreover, early information provisioning aligns with RCOG guidance,52 and other’s recommendations,53 to promote informed decision-making and valid informed consent. However, this kind of information/consent pathway has, to the best of our knowledge, not yet been formally evaluated and careful consideration should be paid to doing this in the event that a future trial goes ahead (e.g. by conducting qualitative work with women and health-care professionals during an inbuilt pilot phase). Consideration should also be given to women’s suggestion that they should receive individualised, rather than generic, information about the trial (i.e. information that is tailored to their specific circumstances, including gestation). Of course, although general information about the trial could be given earlier in the antenatal period, it would only be possible to provide tailored information at the point when women become eligible to participate.
We are hesitant to make claims about the distinctive challenges of recruiting ethnic minority women to the potential trial on the basis of the data we collected, not least because we did not have the opportunity to consult white British women and, therefore, to undertake comparative data analyses. Moreover, different issues emerged in the three groups. Given the range of ethnic minority communities now living in the UK, considerably more complex and wide-ranging challenges might be anticipated than we were able to capture. However, a cross-cutting issue, highlighted by FG participants and health-care professional interviewees, was the challenge of recruiting and consenting women who do not use/speak English as a first language. Both groups highlighted a need for appropriate provisioning (e.g. interpreter services). Such concerns and potential solutions have also been identified by others, such as Hussain-Gambles et al. 54,55 Hussain-Gambles et al. 54,55 have further noted how using professional interpreters and producing written materials (e.g. information sheets) in a variety of languages can be extremely expensive and this has also been seen as a key reason why ethnic minority groups continue to be under-represented in clinical trials. 55 This is an issue that the funder may wish to consider if/when commissioning a future (inclusive) trial and deciding on a funding envelope.
The limitations of our sample of women have already been noted. Our sample of health-care professional interviewees included people working in a range of roles and maternity settings across the UK, and with varying levels of experience. The nature of studies, such as our own, however, is that they tend to attract people with above average interest in either the research topic or research more generally. Our health-care professional interviewees emphasised their personal commitment to trials/research and, indeed, many suggested that colleagues at other sites might not share their enthusiasm. Most participating midwives had research roles/responsibilities and their perspectives may differ from those of exclusively clinical colleagues. In other words, the health-care professionals who took part in this study might hold distinctive views, perhaps being more supportive of research generally, but also more ready to critique the specifics of the HTP in the light of their prior research/trial delivery experiences.
Fundamentally, what our findings suggest is that, were a trial to go ahead, considerable difficulties recruiting and randomising sufficient patients should be anticipated. Likewise, the risk of trial failure, due to under-recruitment, should be taken very seriously. The tensions and difficulties revealed by the research-oriented health-care professionals participating in our study seem likely to be even more profound among less research-invested peers. Women’s concerns will also need careful consideration and management.
These findings are summarised in the following section. Chapter 8 then draws together the findings from the qualitative research with those arising from the survey and Delphi exercise, and presents a series of recommendations for the funder to consider.
Summary of key findings from the qualitative interviews with health-care professionals
Support for the idea of a trial involving mode of birth in preterm birth
-
There was wide acceptance that the current evidence base regarding optimal MoB in PTB was deficient, with interviewees highlighting a need for high-quality (RCT) research evidence to guide practice.
-
Interviewees largely agreed that the proposed trial addressed ‘an important question’, but suggested that running such a trial would present many challenges because of the variation in obstetric cultures and practices across the UK and a widespread lack of personal equipoise.
-
Even with modifications (e.g. changes to inclusion/exclusion criteria), interviewees felt that recruitment into a future trial would be likely to prove challenging.
Factors likely to impinge on support for the trial
-
Interviewees revealed or reported strong preferences regarding MoB in PTB, which they expected to have an impact on recruitment and protocol adherence.
-
MoB preferences appeared to emerge as a result of a variety of factors, including experience, skills and confidence.
-
Some interviewees suggested that skills in managing breech/complex vaginal births had diminished because of a general move towards intervention by CS.
Aspects of the trial viewed as particularly challenging
-
Interviewees recognised that recruiting and randomising to the proposed trial would involve obstetricians shifting away from their MoB preferences.
-
Interviewees noted that the eligibility criteria for the proposed trial were broad and included some scenarios/populations in which this shift would be particularly difficult.
-
Areas where obstetricians had especially strong MoB preferences and, therefore, might find recruitment highly challenging included the following.
-
The extremes of prematurity: at later gestations interviewees strongly favoured the MoB usual in term births (i.e. vaginal birth for cephalic presentations and CS for breech) and at 22–24 weeks’ gestation interviewees felt that few health-care professionals would be willing to perform CS.
-
Breech presentation: interviewees reported a widespread preference for birth by CS, particularly, but not exclusively, at later gestations.
-
Elective PTB: interviewees suggested that health-care professionals would be more likely to favour CS when urgent/timely birth was required.
-
Trial eligibility criteria viewed as less problematic
-
Health-care professionals held less firm and clear-cut preferences for MoB between the extremes of prematurity. However, although interviewees anticipated that they/their colleagues would find randomising less challenging in such situations, some important differences in opinion emerged with regard to the composition/boundaries of this ‘less problematic’ group.
-
Some health-care professionals suggested that they/their colleagues would be comfortable randomising all women between 26 and 32 weeks’ gestation.
-
Others suggested that even within this gestational range they/their colleagues would only be comfortable randomising cephalic presentations.
-
Other pragmatic concerns likely to further affect support for the trial
-
Alongside these concerns about recruiting/randomising (some) women into the trial, interviewees, especially research midwives, highlighted a variety of other practical factors that might compromise support for, or local capacity to deliver, the proposed trial. These factors related to the (in)adequacy of clinical and research resources and skills. Interviewees surmised that substantial investment might be required to resolve these concerns.
-
Health-care professional interviewees – and some women – highlighted the challenges of recruiting and consenting women who do not use/speak English as a first language. Both groups stressed the need for appropriate language provision to ensure equity of access and informed consent.
Chapter 8 Conclusions
Summary of study activities
The overall aim of the CASSAVA project was to determine if a trial to define the optimal mode of PTB could be carried out and, if so, determine what sort of trial could be conducted and how it could best be performed. We aimed to determine the specific groups of preterm women and babies for whom there are uncertainties about the best planned MoB, and if there would be willingness to recruit to, and participate in, a randomised trial to address some, but not all, of these uncertainties.
Specifically, we aimed to determine which of the four statements below is most accurate, and to define any uncertainties:
-
There are no uncertainties about the best-planned MoB for any groups of women or babies presenting in preterm labour.
-
There are uncertainties about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, and in the willingness of clinicians to recruit to, and of women to participate in, a randomised trial to address these uncertainties.
-
There are uncertainties about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, and in the willingness of clinicians to recruit to, and of women to participate in, a randomised trial to address some, but not all of these uncertainties.
-
There is uncertainty about the best-planned MoB for specific subgroups of women (which we will define) presenting in preterm labour, but women and/or clinical staff are not willing to participate in a randomised trial to address any of these uncertainties.
We planned a series of clinician and patient surveys and a consensus workshop, using Delphi methods, to inform the design of a hypothetical clinical trial (HCT). We planned to devise a protocol for the HCT and a vignette for discussion with potential participants. We planned FGs to talk to participants about the trial, and telephone interviews to talk to clinicians. Last, we had planned to design and cost a full trial.
We broadly achieved our aims. We conducted clinician and patient surveys and the consensus workshop, achieving our planned sample size for each. These events were richly informative for the design of a protocol for a HCT (which we called CASSAVAplus) and a vignette for discussion with potential participants. We also reached our planned sample size for in-depth interviews with clinicians.
Unfortunately, in March 2020, 5 months into the qualitative part of this study (with FGs with potential participants and telephone interviews with clinicians), the study sponsor stopped all non-urgent public health research (including the CASSAVA project) as a result of the COVID-19 pandemic. Face-to-face FGs were clearly not going to be possible (because of social distancing requirements). In mid-April 2020, we considered virtual (online) FGs. However, consultation through a participant group (the Bliss Insight and Involvement Group) demonstrated that participants (especially those currently pregnant) might be experiencing heightened anxiety due to the pandemic, which could potentially be compounded by research participation. In a face-to-face setting, this anxiety can be somewhat addressed by a skilled moderator, but this is much more difficult in a virtual setting. Further FGs were, therefore, not conducted, leaving a smaller than planned sample size for the participant FGs (13 participants recruited compared with the planned number of 30–60 patients). Therefore, only tentative and relatively limited conclusions can be drawn about women’s willingness to participate and the particular challenges of recruiting from ethnic minority communities. We did, however, achieve our planned sample size for telephone interviews with clinicians.
Summary of results
Despite the curtailing of the participant FGs, the project has given rich information on the uncertainties around modes of PTB controversies that any trial is most likely to be able to be address. It has also identified potential challenges that will require careful thought in the design and conduct of any substantive trial. The clinician survey demonstrated that there was most variation in practice for babies with a breech presentation in spontaneous labour between 23 and 32 weeks’ gestation, and with indicated PTB (e.g. for fetal growth restriction or pre-eclampsia) with a cephalic presentation of the baby. The parent survey suggested that women and their families generally preferred vaginal birth at later gestations and CS for preterm infants.
Regarding any potential trial, the interactive workshop and Delphi consensus process showed considerable discrepancies in views around the appropriate participants to include. Different views were expressed on the inclusion of, or exclusion of, women with a broad range of numbers of previous pregnancies, body mass index, gestation of planned birth, presentation (breech or cephalic) and multiple pregnancy. In other words, there was no strong feeling that any of these groups should be more focused or excluded. There was lower enthusiasm for including women at very early gestations (e.g. prior to 24 weeks’ gestation) and moderately lower enthusiasm for including women with a previous CS.
Other views that were strongly expressed at the consensus workshop were that women should be spoken to early (i.e. prior to the onset of labour) about the study and that being inclusive (i.e. having wide inclusion criteria) would be best.
Interviews with health-care professionals broadly supported the data provided in the health-care professional survey. The need for more evidence (and, therefore, the case for a trial) was broadly acknowledged. However, many interviewees anticipated that delivery of the proposed trial would present considerable challenges. Interviewees highlighted the existence of strong MoB preferences (i.e. lack of personal equipoise) and noted the potential of these to undermine support for the proposed trial. Preferences were suggested as being most marked at the extremes of prematurity, in breech presentations and in elective delivery scenarios (i.e. in the absence of labour). Preferences were least marked at between 26 and 32 weeks’ gestation and with cephalic presentations and interviewees surmised that they and/or their colleagues would find recruitment and randomisation less problematic in these circumstances. Interviewees also conjectured that allowing individual hospitals or clinicians to modify the inclusion/exclusion criteria within the range allowed by the protocol (in other words, not to recruit particular groups of women, even though the protocol allowed it) might make the trial more widely acceptable to health-care professionals. Some interviewees further suggested that confidence in other aspects of the trial design, such as the utility and robustness of outcome measures, might need attention for the trial to secure widespread support. Finally, interviewees highlighted several other pragmatic issues that might make trial delivery challenging. These concerned the adequacy of local service and research resources, as well as local clinical and research skills (e.g. trial expertise and expertise in all study interventions, including vaginal breech birth).
Although data saturation was not achieved in the FGs, some issues emerged that suggest potentially important concerns about trial participation. FG participants expressed unease about the potential for randomisation to result in a woman having an unnecessary surgery. Participants found the idea of being asked to make such ‘a big decision’ in the intrapartum period deeply problematic. As in the consensus workshop, FG participants overwhelmingly favoured early provision of information prior to the onset of labour. However, some participants also suggested that more personalised information that is tailored to an individual’s particular circumstances, including their stage of pregnancy, should be provided ahead of seeking consent. Additional issues relating to the provision of information and securing of informed consent were identified as potentially important when English was not a woman’s first language.
Planning for a future trial
The CASSAVA project has overwhelmingly demonstrated that there is still a paucity of evidence and lack of consistency in clinical opinion and practice on the optimal MoB for women having a baby preterm, as well as an appetite among the health-care professional community for better evidence to inform their practice. Although previous randomised trials have been attempted and have failed to recruit,3 a clinical trial is likely to be the only way to effectively address this uncertainty. In parallel with the CASSAVA project, a formal update of a systematic review comparing methods of birth is being undertaken (PROSPERO CRD42018097330) and completion of this systematic review is anticipated late in 2021. However, it is not expected that this will address the fundamental uncertainty about the best MoB for women with PTB.
Importantly, previous clinical trials in this area have failed to recruit sufficient participants to determine optimal mode of delivery. CASSAVA has given some helpful indicators about how any trial should be conducted. First, it is clear that potential participants (and clinicians) find the concept of equipoise challenging and that early information for pregnant women on the uncertainties around the best MoB for preterm babies and on the trial would be useful. Second, despite the lack of formal evidence, not all clinicians and not all potential participants, are in equipoise about every clinical situation. Reviewing the inclusion/exclusion criteria and/or allowing clinicians ‘not to recruit’ particular patient groups, in the same way that individual participants are free to choose whether or not to participate, would be important. Third, clinicians and participants would need to have confidence in the trial design, in the resources for the trial and in the trial team to wish to participate. These resources would include written and verbal translation for those whose preferred language is not English, updated teaching in background information, in some methods of birth (e.g. vaginal breech birth) time to spend discussing the trial with women well in advance of them being eligible for recruitment and a local research team to address some of the local burden involved in participating in any trial. Particular issues are likely to be experienced in including women from minority ethnic backgrounds. Various roadmaps56 and toolkits57 could provide useful information and an appropriately funded study within a trial58 might be helpful in determining the most inclusive approach for involving pregnant women from ethnic minorities at risk of PTB. Fourth, either a stand alone pilot/feasibility study (with relevant qualitative work) or one nested within any substantive trial could inform trial procedures, and an adaptive design might address the variety in participant characteristics. Last, although recruitment might be optimised by restricting gestational age of entry and limiting the study setting to units with a co-tertiary neonatal intensive care unit, it may also reduce generalisability.
Given the above issues, and the challenges created by COVID-19, the design and costing of a substantive trial has not been finalised. The outline and detailed design of CASSAVAplus, which we used to consult with potential participants and clinicians, provides a template that can be modified with feedback gained and in the light of any information that emerges from a systematic review with regard to a likely budget envelope.
The CASSAVA project has been crucial in defining what sort of trial could be conducted and how it could best be performed. The CASSAVA project has also outlined the many, significant challenges in conducting such a trial. The chairperson of our Study Steering Committee (Katie Morris) indicated that a qualitative process evaluation nested within a National Institute for Health Research-funded trial, C-STICH2 (Emergency Cervical Cerclage to Prevent Miscarriage and Preterm Birth – a Randomised Controlled Trial; reference 16/151/01), identified similar themes to the CASSAVA qualitative research. C-STICH2 has successfully recruited women to the RCT in this challenging area, but accrual is slow, demonstrating the need for funders to appreciate the difficulties in these areas and demonstrating the need for academics to consider novel methodology.
Summary
We have demonstrated that a trial to determine the best MoB for women having a PTB would indeed be challenging. However, we reject the suggestion that defining the optimal MoB for preterm babies is ‘too difficult’ for research. Around 60,000 (7%) babies are born preterm in the UK each year. MoB is likely to influence the baby’s survival and health in infancy and in later life. Given the huge risks associated with birth (which are not equalled until a person reaches the age of 92 years),59 to fail to define the optimal MoB is unhelpful. The UK has a proud tradition of collaborative research in pregnancy health and we believe that it is time that attention is turned to this most fundamental of questions for preterm babies – what is the best MoB?
Acknowledgements
We are grateful to Lorraine Adamson for excellent administrative support throughout the study.
We are very grateful to the following members of the Study Steering Committee:
-
Professor Katie Morris (chairperson), Consultant Maternal Fetal Medicine, Director Birmingham Clinical Study Unit, University of Birmingham/Birmingham Women’s Hospital.
-
Dr Katie Gillies (methodological research), Health Care Assessment Programme Director, MRC Methodology Research Fellow, University of Aberdeen.
-
Professor Dame Tina Lavender (qualitative research), Professor of Midwifery, University of Manchester/University of Liverpool.
-
Ms Jacqueline Dow (expert patient advisor).
-
Professor Jane E Norman (chief investigator), Professor of Fetal and Maternal Medicine, University of Edinburgh, University of Bristol.
-
Mr Chris Coner (research and development/Academic and Clinical Central Office for Research Development, sponsor representative), Research Governance Co-ordinator, University of Edinburgh.
Last, we are grateful to Islam Gammaledin and Hollie Garbett for their help with the consensus meeting.
Contributions of authors
Jane E Norman (https://orcid.org/0000-0001-6031-6953) (Dean, Faculty of Health Sciences, University of Bristol) conceived the study idea, led the overall design and overall co-ordination of the study, led the parent survey, led the drafting of CASSAVAplus and co-ordinated the drafting of the report.
Julia Lawton (https://orcid.org/0000-0002-8016-7374) (Professor of Heath and Social Science, University of Edinburgh) was a co-investigator, designed and supervised the qualitative research, and was involved in data analysis and the drafting of Chapter 7.
Sarah J Stock (https://orcid.org/0000-0003-4308-856X) (Reader and Subspecialist in Maternal and Fetal Medicine, University of Edinburgh Usher Institute) was a co-investigator, provided obstetric and clinical trial expertise, led the clinician survey and helped draft the report.
Dimitrios Siassakos (https://orcid.org/0000-0002-1078-9856) (Reader, Associate Professor in Obstetrics, Deputy Director of the University College London Wellcome/Engineering and Physical Sciences Research Council Centre for Interventional and Surgical Sciences) co-conceived the study idea, co-led the design, led the Delphi study and provided obstetric input throughout the design and delivery.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Director of the Clinical Trials Unit, University of Edinburgh Usher Institute) contributed to study design and analysis.
Nina Hallowell (https://orcid.org/0000-0002-7647-8524) (Professor and Co-Director of Engineering and Physical Sciences Research Council Centre for Doctoral Training in Health Data Science, University of Oxford) was a co-investigator, provided ethics support for the project, participated in the Delphi exercise and supported some of the FGs and the initial analysis of qualitative data.
Sushila Chowdhry (https://orcid.org/0000-0002-2766-1808) (Lecturer, Health Sciences, University of Dundee) conducted the interviews and FGs, took a lead role in data analysis and drafted sections of Chapter 7.
Ruth I Hart (https://orcid.org/0000-0003-2129-9163) (Research Fellow, Qualitative, University of Edinburgh) contributed to the analysis of the qualitative data and drafted sections of the Chapter 7.
David Odd (https://orcid.org/0000-0002-6416-4966) (Senior Lecturer, Neonatologist, University of Cardiff) provided neonatal input throughout the design and delivery of the project and helped draft the report.
Jane Brewin (https://orcid.org/0000-0002-8411-1802) (Chief Executive of Tommy’s) represented the views and aspirations of parents and Tommy’s, used Tommy’s social media and marketing resources to disseminate surveys to collect people’s opinions and contributed to data interpretation.
Lucy Culshaw (https://orcid.org/0000-0002-4542-2659) (Senior Research Officer, Bliss) represented the views and aspirations of parents, used Bliss’ social media and marketing resources to disseminate surveys to collect people’s opinions and contributed to data interpretation.
Caroline Lee-Davey (https://orcid.org/0000-0003-2132-2378) (Chief Executive, Bliss) represented the views and aspirations of parents, used Bliss’ social media and marketing resources to disseminate surveys to collect people’s opinions and contributed to data interpretation.
Hannah Tebbutt (https://orcid.org/0000-0003-4211-7453) (parent representative) represented the views and aspirations of parents, and contributed to study design and to data interpretation.
Sonia Whyte (https://orcid.org/0000-0003-0878-4244) (Clinical Trials Manager, University of Edinburgh) contributed to study design and oversaw study conduct and appropriate permissions (including ethics).
All authors either drafted the manuscript or revised it critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data-sharing statement
Data on the clinician and patient surveys and anonymised worksheets from the Delphi exercise can be requested from the corresponding author. The qualitative data sets reported on in this publication are not publicly available as rendering them entirely unidentifiable would require significant redaction, and research participants did not consent to public data sharing.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345. https://doi.org/10.1136/bmj.e7976.
- Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012;345. https://doi.org/10.1136/bmj.e7961.
- Alfirevic Z, Milan SJ, Livio S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD000078.pub3.
- National Institute for Health and Care Excellence (NICE) . Preterm Labour and Birth 2015.
- Werner EF, Han CS, Savitz DA, Goldshore M, Lipkind HS. Health outcomes for vaginal compared with cesarean delivery of appropriately grown preterm neonates. Obstet Gynecol 2013;121:1195-200. https://doi.org/10.1097/AOG.0b013e3182918a7e.
- Werner EF, Savitz DA, Janevic TM, Ehsanipoor RM, Thung SF, Funai EF, et al. Mode of delivery and neonatal outcomes in preterm, small-for-gestational-age newborns. Obstet Gynecol 2012;120:560-4. https://doi.org/10.1097/AOG.0b013e318265b16c.
- Reddy UM, Zhang J, Sun L, Chen Z, Raju TN, Laughon SK. Neonatal mortality by attempted route of delivery in early preterm birth. Am J Obstet Gynecol 2012;207. https://doi.org/10.1016/j.ajog.2012.06.023.
- Gamaleldin I, Harding D, Siassakos D, Draycott T, Odd D. Significant intraventricular hemorrhage is more likely in very preterm infants born by vaginal delivery: a multi-centre retrospective cohort study. J Matern Fetal Neonatal Med 2019;32:477-82. https://doi.org/10.1080/14767058.2017.1383980.
- Kuper SG, Sievert RA, Steele R, Biggio JR, Tita AT, Harper LM. Maternal and neonatal outcomes in indicated preterm births based on the intended mode of delivery. Obstet Gynecol 2017;130:1143-51. https://doi.org/10.1097/AOG.0000000000002320.
- Riskin A, Riskin-Mashiah S, Bader D, Kugelman A, Lerner-Geva L, Boyko V, et al. Delivery mode and severe intraventricular hemorrhage in single, very low birth weight, vertex infants. Obstet Gynecol 2008;112:21-8. https://doi.org/10.1097/AOG.0b013e31817cfdf1.
- National Institute for Health and Care Excellence (NICE) . Caesarean Birth 2011.
- Alfirevic Z, Milan SJ, Livio S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev 2012;6. https://doi.org/10.1002/14651858.CD000078.pub2.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet 2000;356:1375-83. https://doi.org/10.1016/S0140-6736(00)02840-3.
- Penn ZJ, Steer PJ, Grant A. A multicentre randomised controlled trial comparing elective and selective caesarean section for the delivery of the preterm breech infant. Br J Obstet Gynaecol 1996;103:684-9. https://doi.org/10.1111/j.1471-0528.1996.tb09838.x.
- Viegas OA, Ingemarsson I, Sim LP, Singh K, Cheng M, Ratnam SS, et al. Collaborative study on preterm breeches: vaginal delivery versus caesarean section. Asia Oceania J Obstet Gynaecol 1985;11:349-55. https://doi.org/10.1111/j.1447-0756.1985.tb00754.x.
- Zlatnik FJ. The Iowa premature breech trial. Am J Perinatol 1993;10:60-3. https://doi.org/10.1055/s-2007-994704.
- Wallace RL, Schifrin BS, Paul RH. The delivery route for very-low-birth-weight infants. A preliminary report of a randomized, prospective study. J Reprod Med 1984;29:736-40.
- Barrett JF, Hannah ME, Hutton EK, Willan AR, Allen AC, Armson BA, et al. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med 2013;369:1295-305. https://doi.org/10.1056/NEJMoa1214939.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.CD004224.pub3.
- Crowther CA, Dodd JM, Hiller JE, Haslam RR, Robinson JS. Birth After Caesarean Study Group . Planned vaginal birth or elective repeat caesarean: patient preference restricted cohort with nested randomised trial. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001192.
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-132.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. London: Sage Publications Ltd; 1990.
- Kitzinger J. The methodology of focus groups: the importance of interaction between participants. Sociol Health Illn 1994;6:103-21. https://doi.org/10.1111/1467-9566.ep11347023.
- Wilkinson CE, Rees CE, Knight LV. ‘From the heart of my bottom’: negotiating humor in focus group discussions. Qual Health Res 2007;17:411-22. https://doi.org/10.1177/1049732306298375.
- Duggleby W. What about focus group interaction data?. Qual Health Res 2005;15:832-40. https://doi.org/10.1177/1049732304273916.
- Higgins JPT, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley; 2019.
- Turner CE, Young JM, Solomon MJ, Ludlow J, Benness C, Phipps H. Willingness of pregnant women and clinicians to participate in a hypothetical randomised controlled trial comparing vaginal delivery and elective caesarean section. Aust N Z J Obstet Gynaecol 2008;48:542-6. https://doi.org/10.1111/j.1479-828X.2008.00923.x.
- Lilford RJ, Jackson J. Equipoise and the ethics of randomization. J R Soc Med 1995;88:552-9.
- Chard JA, Lilford RJ. The use of equipoise in clinical trials. Soc Sci Med 1998;47:891-8. https://doi.org/10.1016/S0277-9536(98)00153-1.
- Garcia J, Elbourne D, Snowdon C. Equipoise: a case study of the views of clinicians involved in two neonatal trials. Clin Trials 2004;1:170-8. https://doi.org/10.1191/1740774504cn020xx.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Hart RI, Hallowell N, Harden J, Jesudason AB, Lawton J. Clinician-researchers and custodians of scarce resources: a qualitative study of health professionals’ views on barriers to the involvement of teenagers and young adults in cancer trials. Trials 2020;21. https://doi.org/10.1186/s13063-019-3942-y.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. https://doi.org/10.1177/1740774510392257.
- Lawton J, Blackburn M, Breckenridge JP, Hallowell N, Farrington C, Rankin D. Ambassadors of hope, research pioneers and agents of change-individuals’ expectations and experiences of taking part in a randomised trial of an innovative health technology: longitudinal qualitative study. Trials 2019;20. https://doi.org/10.1186/s13063-019-3373-9.
- Tong A, Sainsbury P, Craig J. COnsolidated criteria for REporting Qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Royal College of Midwives . Midwifery Care in Labour Guidance for All Women in All Settings 2018.
- Rowland L, Jones C. Research midwives: importance and practicalities. Br J Midwifery 2013;21:60-4. https://doi.org/10.12968/bjom.2013.21.1.60.
- Boulton M, Fitzpatrick R. Evaluating qualitative research. Evid Based Healthc 1997;4:83-5. https://doi.org/10.1016/S1462-9410(05)80078-6.
- Steinke I, Flick U, von Kardorff E, Steinke I. A Companion to Qualitative Research. London: Sage Publications Ltd; 2004.
- Fielding N, Thomas H, Gilber N. Researching Social Life. London: Sage Publications Ltd; 2008.
- Patton M. Qualitative Evaluation and Research Methods. London: Sage Publications Ltd; 2002.
- Ruan G, Bernard H. Techniques to identify themes. Field Methods 2003;15:85-109. https://doi.org/10.1177/1525822X02239569.
- Lincoln YS, Gubba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage Publications Ltd; 1985.
- Kitzinger J, Holloway I. Qualitative Research in Health Care. Maidenhead: Open University Press; 2005.
- O’Dell L, Crafter S, de Abreu G, Cline T. The problem of interpretation in vignette methodology in research with young people. Qual Res 2012;12:702-14. https://doi.org/10.1177/1468794112439003.
- Liamputtong P. Focus Group Methodology: Principles and Practice. London: Sage Publications Ltd; 2011.
- Barbour R, Kitzinger J. Developing Focus Group Research. London: Sage Publications Ltd; 1999.
- Oppenheim M. Baby ‘accidentally decapitated inside mother’s womb’ during delivery. Independent 2018. www.independent.co.uk/news/uk/home-news/decapitated-baby-doctor-mothers-womb-delivery-death-vaishnavy-laxman-tribunal-ninewells-hospital-a8344696.html (accessed 1 September 2021).
- Lawton J, Snowdon C, Morrow S, Norman JE, Denison FC, Hallowell N. Recruiting and consenting into a peripartum trial in an emergency setting: a qualitative study of the experiences and views of women and healthcare professionals. Trials 2016;17. https://doi.org/10.1186/s13063-016-1323-3.
- Royal College of Obstetricians and Gynaecologists . Obtaining Valid Consent 2015.
- Vernon G, Alfirevic Z, Weeks A. Issues of informed consent for intrapartum trials: a suggested consent pathway from the experience of the Release trial [ISRCTN13204258]. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-13.
- Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community 2004;12:382-8. https://doi.org/10.1111/j.1365-2524.2004.00507.x.
- Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P. Involving South Asian patients in clinical trials. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8420.
- Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 2020;21. https://doi.org/10.1186/s13063-020-04613-7.
- National Institute for Health Research Applied Research Collaboration East Midlands . Increasing Participation of Black Asian and Minority Ethnic (BAME) Groups in Health and Social Care Research n.d. http://arc-em.nihr.ac.uk/clahrcs-store/increasing-participation-black-asian-and-minority-ethnic-bame-groups-health-and-social (accessed 2 September 2021).
- National Institute for Health Research . Studies Within a Trial (SWAT) n.d. www.nihr.ac.uk/documents/studies-within-a-trial-swat/21512 (accessed 2 September 2021).
- Walker KF, Cohen AL, Walker SH, Allen KM, Baines DL, Thornton JG. The dangers of the day of birth. BJOG 2014;121:714-18. https://doi.org/10.1111/1471-0528.12544.
Appendix 1 Updated search strategy and outputs from relevant papers to inform the Delphi survey
A search was conducted in MEDLINE using the medical subject headings premature birth AND delivery, obstetric AND randomised trial; premature birth AND caesarean delivery AND randomised trial; premature birth AND labor; obstetric AND randomised trial, from January 2011.
MEDLINE
Date of search: 1 May 2019.
Date range searched: 1 January 1980 onwards.
Search strategy
-
(Preterm).ti,ab
-
(prematur* AND (neonat* OR neo-nat* OR infant OR baby OR babies)).ti,ab
-
exp “INFANT, PREMATURE”/
-
exp “PREMATURE BIRTH”/
-
OR/1-4
-
“mode of delivery”.ti
-
vaginal.ti
-
“trial of labor” OR “trial of labour”.ti
-
(cesarea* OR caesarea*).ti
-
(“c section*”).ti
-
(postcesarea* OR postcaesarea*).ti
-
exp *”CESAREAN SECTION”/
-
*”TRIAL OF LABOR”/
-
OR/6-13
-
(“randomized controlled trial”).pt
-
(“controlled clinical trial”).pt
-
(randomized OR randomly).ab
-
“CLINICAL TRIALS AS TOPIC”/
-
(trial).ti
-
(“control group*”).ti,ab
-
CASE-CONTROL STUDIES/
-
CONTROL GROUPS/
-
MATCHED-PAIR ANALYSIS/
-
(case* ADJ5 control*).ti,ab
-
(case ADJ3 comparison*).ti,ab
-
COHORT STUDIES/
-
LONGITUDINAL STUDIES/
-
FOLLOW-UP STUDIES/
-
PROSPECTIVE STUDIES/
-
RETROSPECTIVE STUDIES/
-
(cohort).ti,ab
-
(longitudinal).ti,ab
-
(prospective).ti,ab
-
(retrospective).ti,ab
-
OR/15-34
-
5 AND 14 AND 35
-
exp ANIMALS/NOT HUMANS/
-
36 NOT 37
-
38 [DT 2015-2017] [Languages English].
Additional scenarios
| Additional scenario | Relevant papers |
|---|---|
| Maternal factors | |
| Body mass index | Tetsuya et al., 2017; Houde et al., 2015; Khalak et al., 2015; Obican et al., 2015 |
| Hypertensive disease | Broekhuijsen et al., 2015; Feghali et al., 2015 |
| Gestational diabetes | Tetsuya et al., 2017; Mardy et al., 2016 |
| Advancing maternal age | Cui et al., 2016; Bereczky et al., 2015 |
| PPROM | Lorthe et al., 2017; Kim et al., 2017 |
| Presence of cervical cerclage | Story and Shannon, 2017 |
| Chorioamnionitis | Lorthe et al., 2017; Feghali et al., 2015 |
| Maternal cardiac condition | Hrycyk et al., 2016 |
| Uterine abnormalities | Cui et al., 2016 |
| Other maternal medical condition | Kim et al., 2017 |
| Placental abnormalities | Tetsuya et al., 2017; Racusin et al., 2016 |
| Fetal factors | |
| Gestational age | Humberg et al., 2017; Thomas et al., 2016; Sentilhes et al., 2015; Holzer et al., 2017 |
| Presentation: breech | Thomas et al., 2016; Bergenhenegouwen et al., 2015; Kayem et al., 2015; Lorthe et al., 2017 |
| Presentation: cephalic | Banister-Tyrrell et al., 2015 |
| Worrying CTG | Racusin et al., 2016 |
| AGA/SGA/LGA | Holzer et al., 2017; Racusin et al., 2016; Chen et al., 2016 |
| Multiple pregnancies | |
| Multiple pregnancies | Hunter et al., 2017; Racusin et al., 2016; Spiegel et al., 2016; Sentilhes et al., 2015 |
| Presentation of twins | Hunter et al., 2017; Spiegel et al., 2016; Sentilhes et al., 2015 |
| Maternal obstetric history | |
| Previous vaginal delivery | Plevani et al., 2016; Mardy et al., 2016; Watson et al., 2017 |
| Previous PTB | Wood et al, 2017; Mardy et al., 2016 |
| Previous CS | Plevani et al., 2016; Hrycyk et al., 2016; Spiegel et al., 2016; Tunc et al., 2016; Rezavand et al., 2016; Mardy et al., 2016; Turitz et al., 2015 |
| Previous CS during second stage | Watson et al., 2017; Levine et al., 2015; Wood et al., 2017 |
| Parity | Lorthe et al., 2017; Kim et al., 2017; Bergenhenegouwen et al., 2015; Feghali et al., 2015 |
Appendix 2 Examples of comments from the Delphi survey
• Parents might not be qualified enough to provide this type of feedback sought at this stage of the study. I am not trained in quantitative methodologies or medical research, therefore, I feel unable to provide a satisfactory answer at the moment.
• The preterm breach is a big problem – the indicated delivery group is too wide in the indication to judge PET [pre-eclampsia] maybe different to just small baby.
• I feel randomised control trials are unethical in all the mentioned scenarios and observational studies would be much more appropriate.
• I found it quite difficult to comment on the scenarios as a parent; rather than a trained professional. I feel that I do not know enough about the risks involved in any of the given scenarios.
• It would be important to join up all scenarios into one or two that are as inclusive as possible.
• Preterm birth of < 24 weeks does not need to be included in the scenarios.
• Disclosure: although I have been invited to complete this as a parent; I am also a GP [general practitioner] I found it difficult to commit to answers to the questions to randomisation of delivery in breech presentation; due to safety concerns regarding delivery of these babies. Does previous history of vaginal delivery need to be added so randomisation is stratified?
• Patients with prolonged ruptured membranes? For inclusion – not easy not know if vaginal birth increases the risk; which may depend on the residual volume of amniotic fluid. Exclude uterine anomalies and know pelvic malformations – I assume the trial will be for singletons only and not twins.
• It would be interesting to hear thoughts on all the presentations of breech – complete; incomplete and frank and whether this changes management – Also women with two previous caesarean sections presenting in preterm labour with a cephalic presentation – It would be good to focus more on singleton pregnancies – so maybe multiple pregnancies as an exclusion.
• Lethal congenital anomalies.
• Exclusion: sepsis; CTG [cardiotocograph] abnormalities; uncertain diagnosis of labour to avoid iatrogenic PTD [preterm delivery] in women with threatened preterm labour who would not have gone on to deliver if in vaginal del arm.
• With the new BAPM [British Association of Perinatal Medicine]/RCOG/RCM [Royal College of Midwives]/Bliss joint draft guidance now talking about resuscitation from 22 weeks – we do need to talk about PTB and outcomes from previable gestation.
• I think it is reasonable to generalise some findings to other scenarios and therefore I am not too sure of the necessity of informing practice with such individual cases.
• I think that the hardest decisions to make are those in spontaneous deliveries; they will also be the hardest to recruit – but those are the patients I’d most like to see in the study!
• I perform ultrasounds and cannot make any comment about other scenario.
• At less than 24 weeks I have said not critical as I would allow a vaginal delivery in these cases and do not think that a CS should take place.
• Not sure how we are meant to know the answers to the questions for different weeks. I can only go on the experience I have.
• Mothers who have been scanned continuously from 20 wks [weeks] are more likely to be more anxious and feel less in control of the birthing plan. Trials on birthing method may help understand which method of delivery would help both mother and child.
• No additional inclusion/exclusion criteria input at present – I continue to follow the progress of the aforementioned study with interest.
• My experience in a clinical situation is not reflective of current practice in our trust but based on my knowledge as a research midwife.
• 28–36 weeks is a very broad span in a potential indicated mode vaginal vs CS – If disease severity enough to warrant delivery at 28 weeks (PE or FGR) [pre-eclampsia or fetal growth restriction] then would be reluctant to wait for an induction. Where does parity come in? Decision-making in multip v different to primip.
Appendix 3 Summary hypothetical trial protocol
FIGURE 17.
Diagram of proposed CASSAVAplus trial. fFN, fetal fibronectin; PROM, premature rupture of membranes.
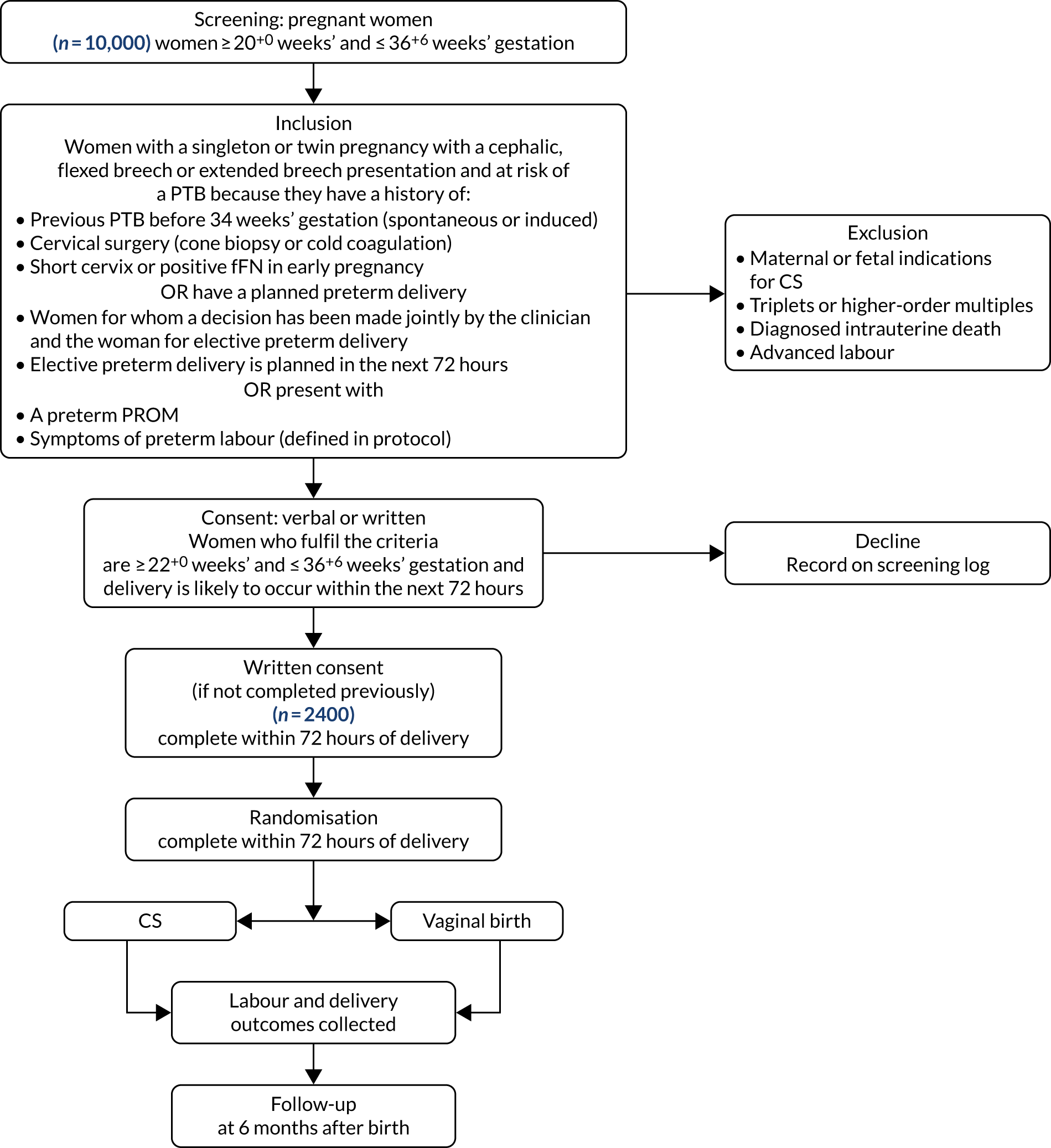
Appendix 4 Full hypothetical trial protocol
List of abbreviations
- 24/7
- 24 hours per day, 7 days per week
- CI
- confidence interval
- CS
- caesarean section
- CSV
- comma-separated values
- FG
- focus group
- GCP
- Good Clinical Practice
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HCT
- hypothetical clinical trial
- HTP
- hypothetical trial protocol
- IVH
- intraventricular haemorrhage
- MoB
- mode of birth
- NICE
- National Institute for Health and Care Excellence
- PG
- pilot group
- PPI
- patient and public involvement
- PTB
- preterm birth
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RR
- relative risk
Notes
-
Questionnaire for clinicians on opinions and practice of mode of birth
-
Summary output from public opinion survey, including free-text comments
-
Guided conversation to inform discussion with health-care professionals
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25610).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
