Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12010. The contractual start date was in September 2013. The final report began editorial review in February 2020 and was accepted for publication in June 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Foy et al. This work was produced by Foy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Foy et al.
SYNOPSIS
Parts of this report are based on Stanworth et al. 1 This is an open access article distributed under the terms of the CC-BY license, which permits unrestricted use, distribution, and reproduction in any medium. You are not required to obtain permission to reuse this article content, provided that you credit the author and journal.
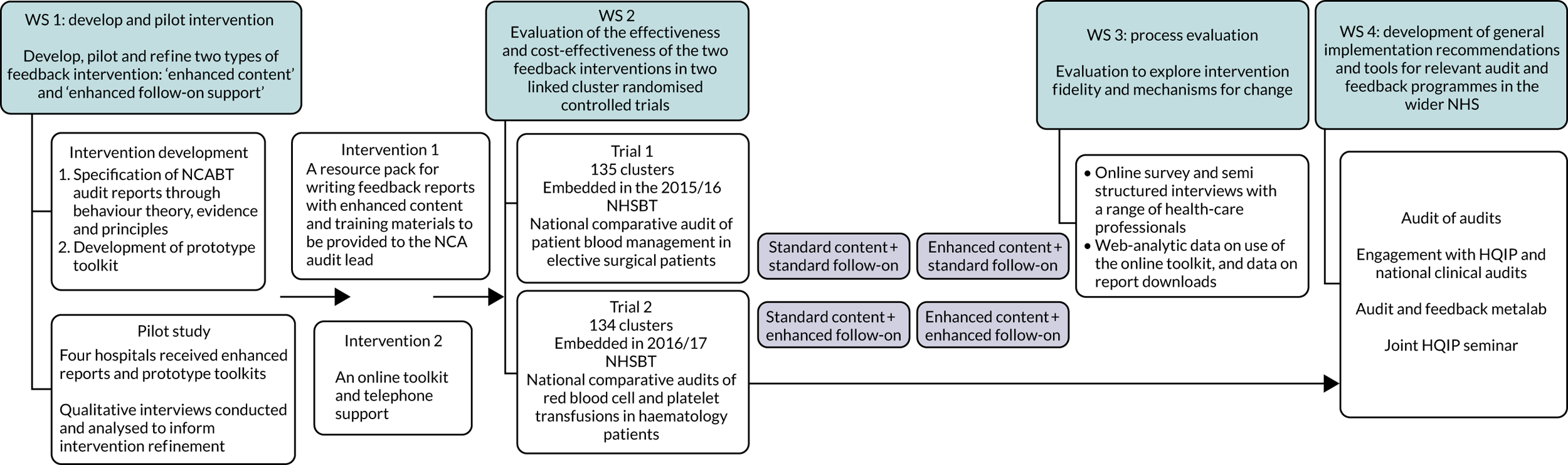
Programme overview
This programme is termed Development & Evaluation of Audit and Feedback INterventions to Increase evidence-based Transfusion practice (AFFINITIE).
We aimed to design and evaluate enhanced feedback interventions, within a national blood transfusion audit programme, to reduce the unnecessary use of blood components. Blood for transfusion is a common intervention in hospital practice. Nearly 2 million issues of blood components are recorded across the UK each year.
The research pathway for the AFFINITIE programme is outlined in Figure 1.
FIGURE 1.
Research pathway diagram.
Text in the following section is reproduced with permission from Hartley et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The AFFINITIE programme of research comprised four workstreams that draw on the UK Medical Research Council (MRC) guidance for developing and evaluating complex interventions. 3 Our objectives were to:
-
develop, pilot and refine two types of feedback intervention – ‘enhanced content’ and ‘enhanced follow-on support’
-
evaluate the effectiveness and cost-effectiveness of the two feedback interventions compared with current standard feedback practice
-
investigate the processes of delivery, including mechanisms of change, for the evaluated interventions
-
develop general implementation recommendations and tools for relevant audit and feedback programmes in the wider NHS. 2
Summary of alterations to the programme’s original aims/design
The analysis presented follows the original proposal, and there were no major alterations. We successfully delivered and tested two behaviourally modified interventions alongside the platform of a national audit. The main trial analysis plan followed that described in the programme’s original aims.
Workstream 1: intervention development and piloting
Background
The National Comparative Audit of Blood Transfusion (NCABT) approach to designing and delivering audit and feedback (A&F) has largely remained unchanged since the organisation was established in 2003. Yet repeated audits have identified high proportions (20%) of unnecessary transfusions,4 raising questions around the effectiveness of the programme’s current approach and whether or not this could be improved. Various theories, in particular control theory (Figure 2), have been used to describe how A&F may operate. 5,6 A number of behaviour change techniques (BCTs)7 are associated with each step in the iterative self-regulatory A&F ‘loop’ described by control theory. 8
FIGURE 2.
Adapted representation of the control theory6 self-regulatory loop with corresponding behaviour change techniques.
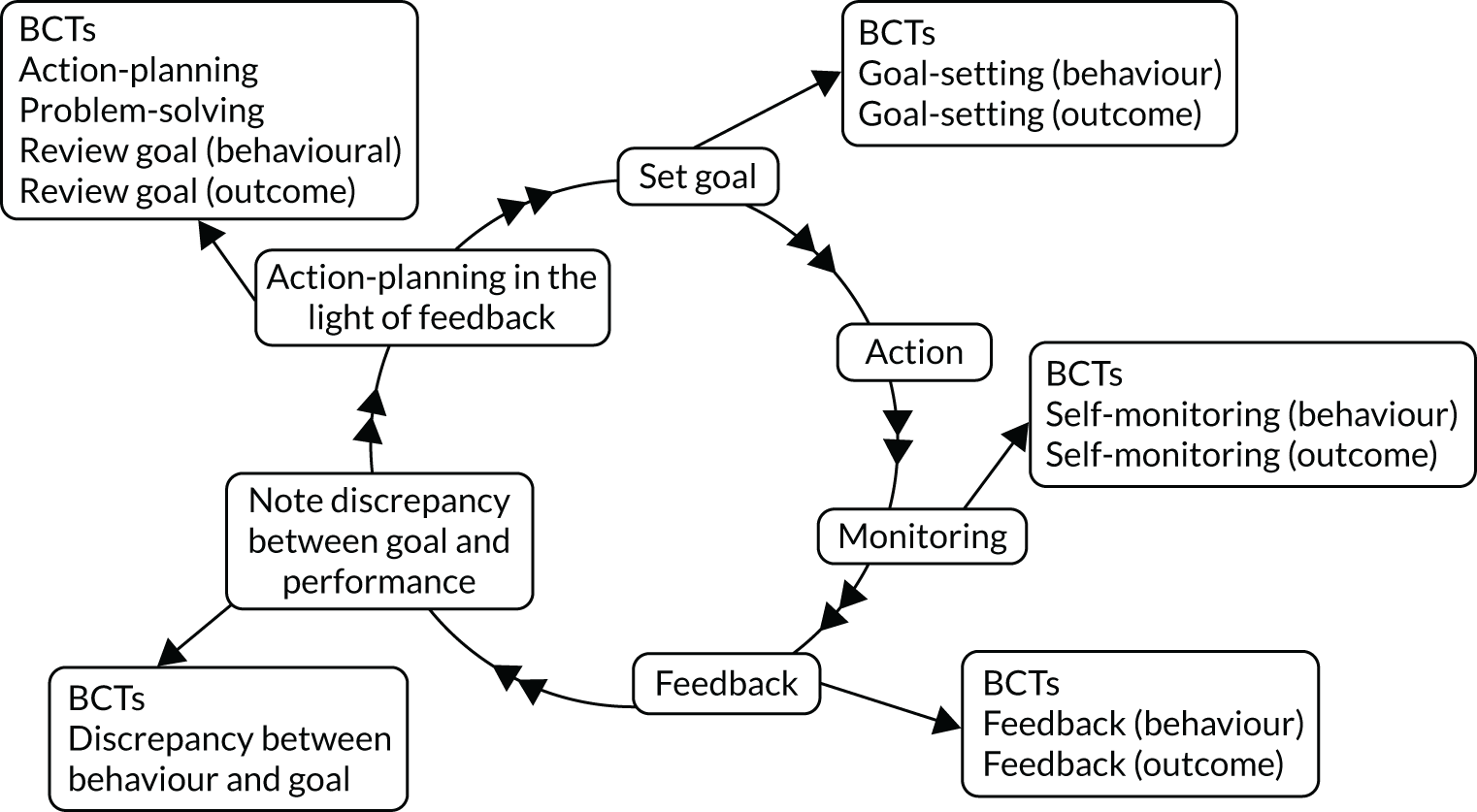
Control theory is consistent with the finding from a Cochrane review9 that A&F is more effective when it is accompanied by explicit goals and action plans. This aligns with behavioural science principles that behaviours are more likely to be enacted if they are explicitly specified in terms of who needs to do what, differently, to whom, where and when [i.e. Actor, Action, Context, Target, Timeframe (the AACTT framework)]. 10
Enhancing existing interventions requires first specifying what is currently done. 11 Investigating current practice can be facilitated through frameworks for identifying, characterising and standardising the reporting of intervention components (i.e. BCT Taxonomy v17) and for investigating factors influencing health-care professional behaviours [i.e. the theoretical domains framework (TDF)]. 12
Aim
We aimed to specify current feedback practice as a basis for developing two theoretically enhanced A&F interventions focused on the design of feedback reports (‘enhanced content’) and supporting hospitals to implement change in the light of feedback (‘enhanced follow-on’), respectively.
The objectives to inform intervention development were to:
-
specify the content of existing A&F reports delivered by the transfusion National Comparative Audit (NCA) and examine the extent to which they include theory-based BCTs8 and evidence-based characteristics9
-
investigate who currently receives feedback from the transfusion NCA, how hospital staff respond to feedback, and the barriers to and enablers of acting on feedback
-
pilot the developed interventions in the hospital context to assess their feasibility and acceptability.
Methods
Based on detailed methods published elsewhere,13 workstream (WS) 1 comprised three mixed-methods sub-workstreams.
Workstream 1a: enhanced content
Design
The workstream design was a structured documentary content analysis.
Materials
We used 12 feedback documents from three recent transfusion NCA audits (four per audit): Red Blood Cells in Neonates and Children 2010 (John Grant-Casey, John Radcliffe Hospital, 2010, personal communication), Platelets in Haematology 2011 (John Grant-Casey, personal communication) and Medical Use of Blood 2012 (John Grant-Casey, personal communication). Documents included summary and full findings reports, action plan templates and presentation slides.
Coding framework
Drawing on available A&F evidence,9 theory6 and behavioural science frameworks,7,10 we developed a coding framework (see Report Supplementary Material 1) to specify current feedback reports in terms of:
-
component BCTs, using the 93-item BCT Taxonomy v17 to identify and categorise BCTs
-
behavioural specificity of each audit standard, feedback item and recommendation, coded according to the AACTT framework10
-
seven evidence-based feedback characteristics, namely whether or not feedback was provided in multiple formats; was delivered by respected peers; was delivered more than once and closely following audit data collection; included explicit goals and action plans; targeted behaviours where baseline performance was low; included multiple comparators (e.g. achievable benchmarks, top 10%); and was supportive rather than punitive in tone. 9
Procedure and analysis
The framework was applied to code each document, with three reports (one per audit) double-coded by two researchers. Inter-rater reliability was assessed using Cohen’s kappa. 14 For each document, we assessed the presence or absence of all 93 BCTs in the taxonomy. We assessed whether 11 BCTs consistent with control theory (see Figure 2) were present or absent in each document. We calculated the proportion of audit standards, feedback items and recommendations for which AACTT were specified. We investigated how many of the eight evidence-based feedback characteristics were identified across documents from each of the three audits.
Intervention development
Evidence- and theory-based feedback components that were absent or identified infrequently were considered possible enhancements to feedback content. These enhancements were incorporated into prototype feedback reports and a ‘how to’ guidance manual for the transfusion NCA for designing feedback reports with theory- and evidence-based content. The WS1a findings, the potential feasibility, and the theoretical and clinical face validity of proposed enhancements were reviewed at a multidisciplinary workshop with behavioural scientists, clinicians, and PPI representatives.
Workstream 1b: enhanced follow-on
Design
We took a multiple case study approach, using semistructured qualitative interviews and observations of hospital transfusion committee meetings. 15
Participants
Four hospitals in England that routinely participate in audits by the transfusion NCA were purposively sampled for variation in infrastructure (e.g. district vs. teaching hospitals). We purposively recruited five to eight health-care professionals from each hospital from roles involved in prescribing and administering transfusions, and implementing change in response to feedback.
Materials
The interview topic guide (see Report Supplementary Material 2) investigated how feedback is operationalised in hospitals, including four key behaviours corresponding to the second half of control theory: dissemination of reports, local goal-setting, action-planning and problem solving, and re-monitoring. Questions exploring the barriers to and enablers of acting on feedback were structured around 12 domains of the TDF16 (e.g. knowledge, social influences, beliefs about consequences, environmental context and resources). We developed an observation sheet (see Report Supplementary Material 3) to record field notes in Hospital Transfusion Committee meetings on when and how A&F was discussed, communication, staff engagement and group decision-making.
Procedure
Interviews were conducted one to one with consenting participants, and then audio-recorded and transcribed verbatim. Two researchers observed one Hospital Transfusion Committee meeting at each hospital, with the consent of the meeting attendees, and recorded field notes.
Analysis
Interview transcripts were analysed using combined deductive framework (based on the TDF) and inductive thematic analysis. 17 The key domains were identified by considering frequency (≥ 60% participants) and participants’ expressed importance. 17 Field notes were summarised thematically. All themes were reviewed by three researchers until consensus was reached. Each case (hospital) was analysed separately. Case 1 was first analysed in full, with data from subsequent hospitals (cases 2–4) analysed by using themes from previous cases while allowing new themes to emerge.
Intervention development
We selected potential BCTs to address the identified barriers/enablers within key domains by consulting matrices that map BCTs from Taxonomy v1 against domains from the TDF,3,18 plus those BCTs consistent with control theory. These were incorporated into a prototype toolkit, including resources to support hospitals to disseminate feedback, set goals, action-plan/problem-solve, and re-monitor. The proposed BCTs and toolkit were discussed at the same multidisciplinary workshop as described for WS1a.
Workstream 1c: feasibility piloting
Four hospitals in England consented to participate in a pilot audit on the medical use of blood. Data were extracted from patient notes based on the audit standards from the 2012 Medical Use of Blood audit (John Grant-Casey, personal communication) and used to develop enhanced feedback reports using the prototype templates. Two researchers independently coded the draft enhanced report and toolkit to ensure that the intended BCTs and enhancements were present (i.e. delivered with fidelity). The prototype enhanced feedback reports and toolkit were then delivered and explained by two members of the research team to the hospital transfusion team during a 1-hour face-to-face training session.
Three months later, qualitative interviews were conducted with staff at each hospital. Staff were purposively sampled as per WS1b. We conducted one-to-one semistructured and think-aloud19 type interviews, which commenced by asking participants if they recalled receiving the feedback reports or toolkit. If yes, a semistructured interview was conducted to explore feasibility and acceptability of these. 20 If no, we conducted a think-aloud interview, whereby staff were presented with the reports and toolkit and asked to verbalise their immediate reactions to the interventions. Both topic guides are available in Report Supplementary Material 4 and 5.
Interviews were audio-recorded, transcribed verbatim and analysed using thematic analysis. The WS1 research team (FL, NG, JF, LG and SJS) then considered potential ways of refining the interventions to address any themes indicating threats to feasibility and acceptability (e.g. adding or removing components, modifying format and modes of delivery). Potential refinements were then discussed at a wider multidisciplinary meeting to reach consensus on which to implement.
Key findings
Workstream 1a: enhanced content
Average inter-rater coding reliability was high (κ = 0.81, range 0.80–0.96). Overall, existing feedback documents incorporated a limited number of theory-and evidence-based components.
Behaviour change techniques
Documents contained on average 8 of the 93 BCTs in Taxonomy v1 (range 3–14) (see Report Supplementary Material 6). They included, on average, half (5.6, 51%) of the 11 BCTs associated with control theory (range 2–7). The most frequent, identified in at least one document from each of the three audits, corresponded to the first half of the control theory loop: ‘goal-setting’ (i.e. audit standards), ‘feedback on behaviour’ and/or ‘outcomes of behaviour’ and ‘discrepancy between behaviour and goal’. Only one BCT, ‘action-planning’, from the second ‘adaptive response’ half of control theory was identified from all three audits (see Report Supplementary Material 6). There were no identified BCTs encouraging review of audit standards, setting of localised goals or ongoing self-monitoring.
Behavioural specificity
Overall, behavioural specificity of audit standards, feedback and recommendations for change was low. Although the action (e.g. measuring pre-transfusion haemoglobin) and target group (e.g. neonates) were often stated, the actor (e.g. consultant haematologists), context (e.g. ‘paediatric ward’) and time (e.g. within 3 days of transfusion, preferably same day) were rarely stated (see Report Supplementary Material 7). Across documents, on average only half (range 0–100%) of the feedback related to behaviours specified in audit standards, indicating that there was a high volume of extraneous feedback.
Evidence-based characteristics
We identified only two of the eight evidence-based feedback characteristics (see Report Supplementary Material 8). Feedback was always provided electronically, in writing, and, rarely, also graphically or using other modalities. The stated feedback provider was always a regulatory body, rather than a respected peer. Feedback was delivered only once, typically 12 months after data collection. Average national performance in relation to audit standards was 72% (range 64–86%), suggesting that baseline performance for targeted behaviours was reasonably high, with limited room for improvement. Although the feedback provided was not necessarily punitive, supportive BCTs such as ‘social reward’ and ‘social support’ were delivered infrequently.
Although the two evidence-based characteristics ‘include explicit goals and action plans’, and ‘use multiple comparators’ were identified from all three audits, these were not fully operationalised. Documents included goals, and some recommendations for change, but these were not explicit, as evidenced by the limited AACTT specification. All audits provided comparative feedback on peer performance (nationally and/or regionally). However, no audit compared practice against achievable benchmarks of care (see Report Supplementary Material 8).
On this basis, six recommendations for enhancing the content of feedback reports were identified and agreed with multidisciplinary stakeholders:
-
include at least one BCT corresponding to each step of control theory
-
‘be specific’ – phrase audit standards, feedback and recommendations in terms of who/what/where/when
-
‘be relevant’ – include only feedback related to audit standards and take a graded-entry approach by producing feedback reports with varying levels of detail, from key findings to supplementary reports
-
include multiple comparators
-
include positive messages recognising good practice
-
improve feedback document presentation (e.g. provide feedback visual format, such as graphs, make writing legible, use a consistent layout, personalise feedback).
Enhanced prototype reports and the enhancement guidance manual are available in Report Supplementary Material 9a–c and Report Supplementary Material 10a–b.
Workstream 1b: enhanced content
Full results are published elsewhere. 15 In summary, we interviewed 25 participants, including nurses, junior doctors, registrars and consultants from haematology and other clinical specialties, and quality improvement managers. 15
Who receives feedback?
Report Supplementary Material 11 depicts the dissemination pathway of feedback reports for each hospital. Dissemination was a key barrier to implementing feedback across all hospitals. Feedback was often initially received by the hospital transfusion team, but then not disseminated more widely to clinical staff from other specialties who prescribe transfusions or to more junior staff. The extent to which feedback from the NCA was discussed at hospital transfusion committee meetings also varied. 15
What do hospitals do with feedback and what are the barriers and enablers?
There was considerable variation in how feedback was received, shared and responded to across hospitals. The key barriers to and enablers of feedback across all cases fell into eight theoretical domains (see Report Supplementary Material 12):
-
social influences – not sharing and discussing feedback with colleagues; lack of influence over practice change and support from peers; desire for feedback to be delivered from a familiar, respected colleague; the view that comparison against national averages is not useful for identifying areas for improvement
-
behavioural regulation – not setting goals and action plans as a team; need for support materials and tools to facilitate planning locally; having to amend feedback to make it relevant locally
-
social professional role/identity – lack of clarity about who is responsible for A&F
-
knowledge – variable and limited awareness of transfusion NCA
-
motivation – competing priorities and audit fatigue
-
environmental context and resources – lack of staff and resources to conduct re-audits and/or implement change
-
beliefs about consequences – A&F does change practice
-
memory attention decision-making – not recalling the feedback materials; noticing new information only when it is different or clinically relevant. 15
The prototype enhanced follow-on intervention included 19 BCTs targeting these barriers and enablers. Report Supplementary Material 13 presents the full list of BCTs and associated TDF domains. These BCTs were delivered through a paper-based ‘toolkit’ to support staff in planning their response to feedback. Tools included a dissemination cascade to facilitate the sharing of feedback reports, a fishbone problem-solving worksheet, goal-setting and action plan templates, and ‘QuickAudit’ local re-monitoring template (see Report Supplementary Material 14). The intervention involved a 1-hour training visit during which a member of the research team met with each hospital transfusion team to talk them through the toolkit (i.e. its purpose and how to use it).
Workstream 1c: feasibility and acceptability
All intended BCTs and enhancements were identified in the prototype feedback reports and toolkit, indicating that these were feasible to embed in intervention materials with fidelity. Twelve staff members participated in semistructured interviews and 14 in think-aloud interviews, across all health-care professional groups (consultant haematologists, geriatricians, gastroenterologists and obstetricians; transfusion practitioners; laboratory managers; nurses; midwives; clinical audit and effectiveness managers).
Six overarching feasibility and acceptability themes were identified for both interventions (see Report Supplementary Material 15 and 16):
-
comprehensibility (i.e. clarity of the content and formatting of the reports and toolkit)
-
preference (i.e. whether or not staff liked the different intervention materials and their preferences for different modes of delivery)
-
usability (i.e. the perceived ease of use and utility of the interventions)
-
engagement (i.e. the extent to which feedback and tools are engaging and capture recipients’ attention)
-
intention (i.e. how likely staff are to read/share the reports and use the toolkit)
-
effectiveness (i.e. the likely impact that the interventions have on practice).
Responses from participants, including health-care professionals, within these six overarching themes were mostly positive (e.g. enhanced content: ‘the recommendations for my hospital are clear, general findings for clinical staff, different groups . . . it lays out quite clearly what we’ve got to do’; enhanced follow-on: ‘as I’m going through I think it’s a really good resource for the teams . . . as a refresher or a reminder or even if they’re not doing it properly in the first place’). We therefore made no refinements to the intervention.
Further improvement suggestions were recorded, including modifiable changes to the formatting and design of the report (e.g. font type, size, colour). Time was identified as a key barrier to feasibly delivering the toolkit in the trial in terms of the availability of hospital transfusion staff to attend the toolkit training session and researchers travelling to sites nationally to deliver this. Participants did not find the paper-based tools sufficiently engaging, interactive or facilitative of team working. Two major refinements were therefore made. First, as proposed by participants, the mode of delivery was changed to web based, which supported greater interactivity and data-sharing among colleagues. Second, the in-person training session was replaced with a telephone support co-intervention, whereby staff were prompted to log in to the toolkit and each tool was explained and demonstrated. A telephone helpline was made available for the duration of the trial. Final intervention materials are available in Report Supplementary Material 9a–c, 10a–b, 15 and 16. A Template for Intervention Description and Replication (TIDieR) intervention specification checklist is available in Report Supplementary Material 17. 21
Conclusions
Current blood transfusion A&F in England makes limited use of the available theory and evidence about how to effectively design and deliver A&F. We developed two theory- and evidence-enhanced feedback interventions for evaluation in two national cluster-randomised trials (WS2 and WS3).
Limitations
The main limitation is the sample size of feedback documents, hospitals and health-care professionals analysed/participating in each sub-workstream. Although these sources were purposively sampled to ensure maximum diversity, findings from these may not reflect the full variation in practice across hospitals nationally. Qualitative interviews in WS1b and WS1c were also likely to be subject to social desirability and recall biases.
Workstream 2: trials 1 and 2 and cost-effectiveness analysis
Workstream 2a: trial 1
Study design and setting
We conducted a 2 × 2 factorial, cross-sectional, cluster-randomised controlled trial in elective surgical patients, embedded in the 2015–16 NHS Blood and Transplant (NHSBT) NCAs of patient blood management. The two feedback interventions were directed at clinical teams in hospital trusts and health boards across the UK, so randomisation at the cluster level was essential. Different patients were audited at baseline and follow-up, leading to a cross-sectional design. We adopted a 2 × 2 factorial design in which an enhanced content intervention was compared with the standard content intervention across levels of follow-on, and an enhanced follow-on intervention was compared with the standard follow-on intervention across levels of content. Although we recognised that there might be a small antagonistic interaction between the feedback interventions, interest was in the marginal (i.e. main) effects of those interventions, even in the presence of realistic interactions.
All trusts and health boards in the UK were invited to take part, with NHS permissions sought for eligible sites. We assessed outcomes for audited patients of randomised clusters at 12 months after cluster randomisation. In addition, we requested safety data from the Serious Hazards of Transfusion (SHOT) database and blood usage data from the Blood Stocks Management Scheme (BSMS) database.
Cluster eligibility
We based eligibility on the following:
-
inclusion criteria –
-
providing an NHS service relevant to the audit topic
-
accepting invitation by the NCA to participate in the audit.
-
-
exclusion criteria –
-
independent hospitals (as clinicians involved in transfusion decisions are likely to practice in multiple clusters, leading to potential contamination)
-
participating in the development of the interventions (also to prevent contamination).
-
Where at least one NCA hospital site in a cluster was eligible, we regarded the cluster as eligible.
Randomisation
The trial statistician undertook randomisation at a single point following receipt of the baseline audit database from the NCA. We randomised trusts or health boards on a 1 : 1 : 1 : 1 basis using a computer-generated minimisation program, incorporating a random element, balancing for trust size (large, medium or small) and regional transfusion committee. We randomised clusters to one of four feedback interventions: (1) standard content/standard follow-on, (2) standard content/enhanced follow-on, (3) enhanced content/standard follow-on or (4) enhanced content/enhanced follow-on. If trusts or health boards merged following randomisation, we continued to regard them as distinct clusters for intervention, data collection and analysis purposes.
Interventions
Current practice was the standard feedback delivered by the NCA following completion of an audit. Feedback is in the form of a written clinical audit-specific report to hospital sites, a regional Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation and often action plan templates. Responses by clinical teams to receipt of feedback is not standardised. We requested that staff who received the feedback did not share with colleagues external to their own trust or health board.
Enhanced content
Enhanced content comprised feedback documents with content written to specifically deliver behaviourally specified feedback and the relevant theoretically consistent BCTs. These were delivered by the NCA programme via written and graphic feedback presented in multiple feedback documents and presentations.
Enhanced follow-on
Enhanced follow-on made use of targeted dissemination of feedback to relevant staff with discussion and agreement of action plans. Follow-on support comprised practical guidance for clinical teams on how to operationalise the process of responding to feedback, including materials for clinical teams to facilitate discussion and agreement of locally relevant goals and action plans based on feedback.
Monitoring intervention adherence
The assessment of fidelity was based on a fidelity framework proposed by the National Institutes of Health (NIH) Behaviour Change Consortium22 to investigate and report the extent to which the enhanced and standard feedback interventions were designed, trained, delivered, received and enacted as intended.
Data collection
Table 1 summarises the required data and collection time points. Baseline was from October 2014 to September 2015 and follow-up was from November 2015 to October 2016. We obtained data from NHSBT, SHOT and BSMS, supplemented with our own collection of trial process data (i.e. withdrawals and fidelity).
| Data (including who provides these data) | Screening | Timeline | |
|---|---|---|---|
| Baseline | Follow-up | ||
| Data regarding trusts/health boards | |||
| Cluster-level screening information | X | ||
| Confirmation of cluster eligibility for the NCA | X | ||
| NHS permissions | X | ||
| Blood stock management | X | X | |
| SHOT reportable events | X | X | |
| Cluster withdrawal | Throughout the trial evaluation | ||
| Clinical audit data | |||
| Clinical audit cases | X | X | |
| Organisation survey | X | X | |
| Data on intervention delivery | |||
| Intervention fidelity (design) | X and Y | ||
| Intervention fidelity (training) | X and Y | ||
| Intervention fidelity (delivery) | X and Y | ||
| Intervention fidelity (receipt) | X and Y | ||
| Intervention fidelity (enactment) | X and Y | ||
| Contamination events | Z | ||
| Unblinding events | Z | ||
| Data on intervention costs | |||
| Resource inputs for audit data collection and submission | X | ||
| Resource inputs for production and delivery of feedback documents | X | ||
| Resource inputs for ‘follow-on support’ intervention | X and Y | ||
Outcomes
Text in the following section is reproduced with permission from Hartley et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Transfusion may occur preoperatively, intraoperatively or postoperatively. There may also be multiple transfusion episodes after surgery but prior to discharge. The prespecified primary outcome, measured at the patient level and taken from the NCA follow-up audit, was whether preoperative and/or first postoperative transfusions were categorised as acceptable or any preoperative or first postoperative transfusions were outside guidelines (binary). 2 A clinical algorithm (see Appendix 1) was agreed by an independent panel, minimising the risk of detection bias, based on clinical relevance and baseline compliance.
Prespecified secondary outcomes comprised:
-
total volume of allogeneic RBCs transfused (units at trust level, from BSMS summed over blood groups and clinical specialties)
-
total volume of allogeneic RBCs transfused (units at patient level, from NCA summed over preoperative, intraoperative and postoperative periods)
-
total number of incidents reported to SHOT (count at trust level, from SHOT summed over clinical specialties and events, near-misses and ‘right blood right patient’ incidents)
-
number of definitely, probably or possibly preventable incidents reported to SHOT within clinical specialties targeted by the audit (count at trust level, from SHOT summed over events, near-misses and ‘right blood right patient’ incidents).
Prespecified supportive outcomes comprised:
-
preoperative transfusion (acceptable/outside guidelines)
-
postoperative transfusion (acceptable/outside guidelines)
-
individual NCA audit standard met (1, 2, 3, 4 and 8; yes/no)
-
preoperative volume of allogeneic RBCs transfused (units at patient level)
-
postoperative volume of allogeneic RBCs transfused (units at patient level)
-
total volume of RBCs issued (units at trust level, from BSMS data)
-
total volume of RBCs transfused (units at trust level, from BSMS data).
All other outcomes (i.e. intermediate NCA outcomes, number of relevant near-miss or ‘right blood right patient’ SHOT incidents, total number of unpredictable SHOT incidents, total number of possibly preventable SHOT incidents and BSMS total volume of RBCs wasted) reported were exploratory.
Sample size
There were two comparisons of interest (enhanced vs. standard content and enhanced vs. standard follow-on), relating to the two main effects of the factorial design under effect coding (–1,1 rather than 0,1). Assuming that 20% of transfusions are outside guidelines at follow-up, that the intracluster correlation coefficient will be 0.05 and that cluster sizes will vary from 17 to 68 with a mean of 45, we required 152 clusters to detect a minimally important reduction of 6% (to 14%) in the presence of, at most, a small antagonistic statistical interaction (i.e. main effects of 5%) with 80% power using logistic regression models, with a random-intercept for cluster, and a two-sided 2.5% significance level, for each comparison.
Statistical analysis
No interim analyses were planned or conducted. Wherever possible, we undertook primary data summaries and analyses on the intention-to-treat (ITT) sample, defined as all randomised clusters analysed as randomised. The proportion of missing data was anticipated to be non-trivial. Therefore, mechanisms for missing data on key variables were explored and multiple imputation was used based on 100 imputations and the full imputation model, under the assumption that data were missing at random (MAR). Sensitivity analyses assessed whether or not the conclusions were robust across approaches to handling missing data. A random intercept model accounted for clustering arising from cluster randomisation, as model convergence was unreliable with more complex structures. Reflecting interest in each main effect, an overall two-sided 5% significance level was used; 97.5% CIs are also presented for the main effects, according to a Bonferroni multiplicity adjustment.
We compared the primary outcome using multilevel logistic regression, adjusting for design factors (trust size, regional transfusion committee) and trust-level proportion of acceptable transfusions at baseline, with effect-coded contrasts for enhanced versus standard content, enhanced versus standard follow-on and their interaction. The patient-level secondary end point of volume of blood transfused is reported descriptively, as are the trust-level secondary outcomes (volume transfused, SHOT incidents). The supportive outcomes act as sensitivity analyses for primary and secondary outcome analyses. We conducted analyses in the statistical package software SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration).
Results
Screening and recruitment
Prior to cluster randomisation (15 October 2015), we screened 189 NHS trusts and health boards. A total of 152 (80.4%) were included in the audit, and 135 of these (88.8%) were randomised. Reasons for exclusion, when given, were mainly mergers or ineligibility, so the included clusters were typical of the UK as a whole. The baseline audit comprised a total of 2714 patients, 40% of target; 66 clusters were allocated to standard content, 69 were allocated to enhanced content, 67 were allocated to standard follow-on, and 68 were allocated enhanced follow-on. An average cluster size of 20 patients was observed, with a coefficient of variation of 0.7 (Figure 3).
FIGURE 3.
Trial 1: NHS trust/health board and patient CONSORT flow diagram.
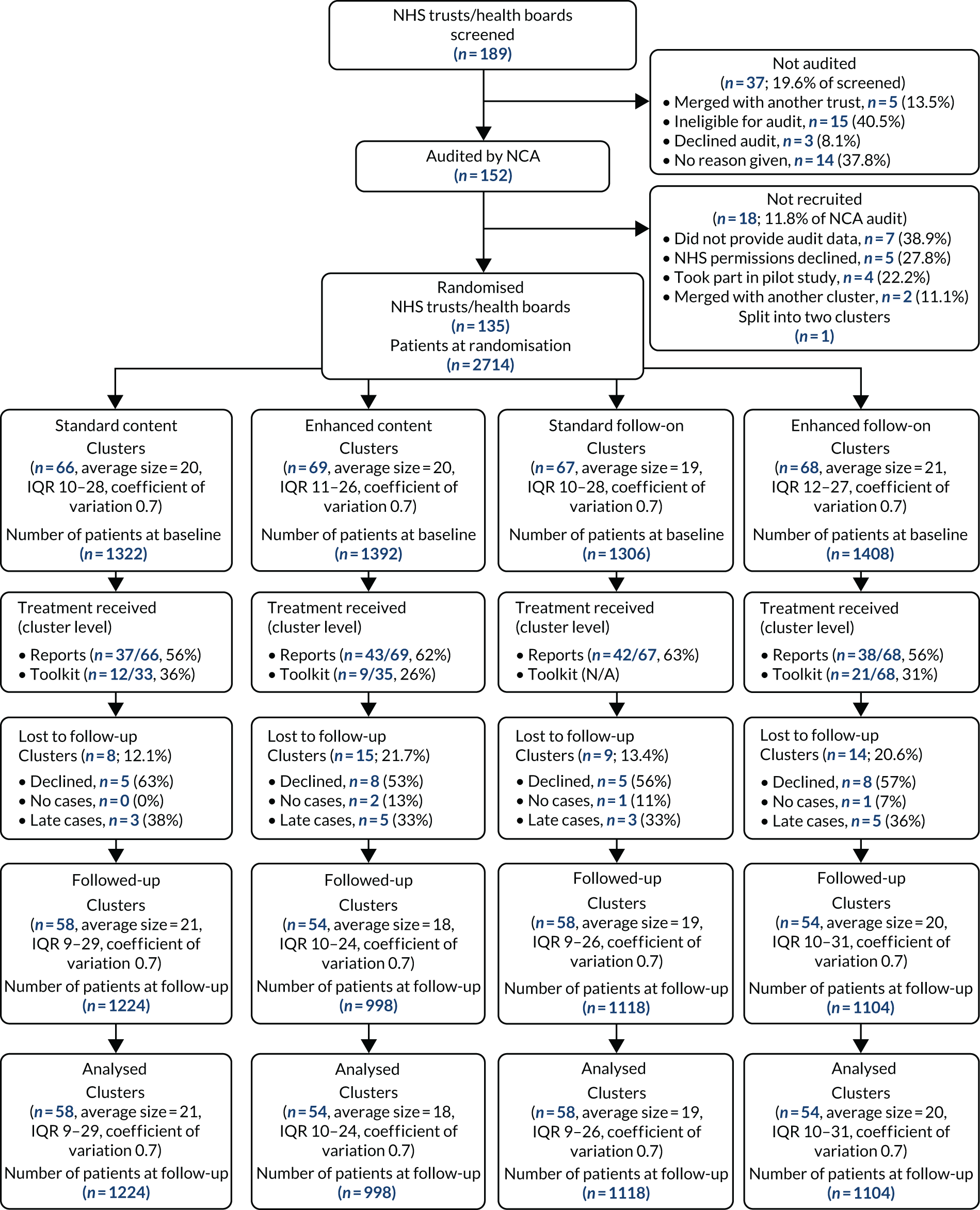
Over half of the clusters received the enhanced (62%) and standard reports (56%). Receipt of the toolkit was lower (31% overall), with more clusters allocated to standard content receiving the toolkit (36%) than those allocated to enhanced content (26%). A total of 23 out of 135 clusters (17%) were lost to follow-up, this proportion being higher among those allocated to enhanced content (22% vs. 12%) and enhanced follow-on (21% vs. 13%). Overall, two clusters had no cases to audit, eight clusters provided audit cases but did so too late, and 13 declined to take part. Among the 112 clusters taking part in the follow-up audit, there were a total of 2222 audit cases (32% of target). Again, an average cluster size of 20 patients and a coefficient of variation of 0.7 were observed. We included all 112 clusters and 2222 patients in our primary analyses. Table 2 shows baseline patient-level characteristics. (Other baseline summaries are given in Appendix 2, Table 10.) These were generally well balanced, as would be expected given that patients were ascertained prior to randomisation. Notably, 30% surgical procedures were for a fractured neck of femur and, as anticipated, these patients would not typically be able to attend a preoperative clinic. In total, 249 (9%) received a preoperative, 363 (13%) an intraoperative and 2560 (94%) at least one postoperative transfusion.
| Variable | Content | Follow-on | Total (N = 2714) | ||
|---|---|---|---|---|---|
| Standard (N = 1322) | Enhanced (N = 1392) | Standard (N = 1306) | Enhanced (N = 1408) | ||
| Age (years), mean (SD), n | 74.7 (13.80), 1318 | 75.1 (14.13), 1383 | 75.3 (13.80), 1302 | 74.6 (14.12), 1399 | 74.9 (13.97), 2701 |
| Gender (male), n (%) | 435 (32.9) | 470 (33.8) | 418 (32.0) | 487 (34.6) | 905 (33.3) |
| Surgical procedure, n (%) | |||||
| Orthopaedic | 444 (33.6) | 484 (34.8) | 435 (33.3) | 493 (35.0) | 928 (34.2) |
| Cardiac | 233 (17.6) | 222 (15.9) | 222 (17.0) | 233 (16.5) | 455 (16.8) |
| Fractured neck of femur | 421 (31.8) | 418 (30.0) | 410 (31.4) | 429 (30.5) | 839 (30.9) |
| Other | 222 (16.8) | 258 (18.5) | 234 (17.9) | 246 (17.5) | 480 (17.7) |
| Missing | 2 (0.2) | 10 (0.7) | 5 (0.4) | 7 (0.5) | 12 (0.4) |
| Attendance at preoperative clinic, n (%) | 839 (63.5) | 922 (66.2) | 841 (64.4) | 920 (65.3) | 1761 (64.9) |
| Surgery complications, n (%) | 328 (24.8) | 381 (27.4) | 326 (25.0) | 383 (27.2) | 709 (26.1) |
| Patient died, n (%) | 49 (3.7) | 63 (4.5) | 61 (4.7) | 51 (3.6) | 112 (4.1) |
| Preoperative transfusion, n (%) | 120 (9.1) | 129 (9.3) | 114 (8.7) | 135 (9.6) | 249 (9.2) |
| Intraoperative transfusion, n (%) | 179 (13.5) | 184 (13.2) | 171 (13.1) | 192 (13.6) | 363 (13.4) |
| Postoperative transfusion, n (%) | 1245 (94.2) | 1315 (94.5) | 1235 (94.6) | 1325 (94.1) | 2560 (94.3) |
| Preoperative blood transfusions | |||||
| N | 120 | 129 | 114 | 135 | 249 |
| Professional making the decision to transfuse, n (%) | |||||
| Nurse | 1 (0.8) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (0.4) |
| Consultant | 55 (45.8) | 50 (38.8) | 47 (41.2) | 58 (43.0) | 105 (42.2) |
| Other doctor | 51 (42.5) | 64 (49.6) | 50 (43.9) | 65 (48.1) | 115 (46.2) |
| Other | 7 (5.8) | 1 (0.8) | 5 (4.4) | 3 (2.2) | 8 (3.2) |
| Missing | 6 (5.0) | 14 (10.9) | 11 (9.6) | 9 (6.7) | 20 (8.0) |
| Number of units transfused, n (%) | |||||
| One | 26 (21.7) | 26 (20.2) | 19 (16.7) | 33 (24.4) | 52 (20.9) |
| Two or more | 93 (77.5) | 101 (78.3) | 94 (82.5) | 100 (74.1) | 194 (77.9) |
| Missing | 1 (0.8) | 2 (1.6) | 1 (0.9) | 2 (1.5) | 3 (1.2) |
| Postoperative blood transfusions | |||||
| N | 1245 | 1315 | 1235 | 1325 | 2560 |
| Professional making the decision to transfuse, n (%) | |||||
| Nurse | 2 (0.2) | 6 (0.5) | 6 (0.5) | 2 (0.2) | 8 (0.3) |
| Consultant | 288 (23.1) | 376 (28.6) | 289 (23.4) | 375 (28.3) | 664 (25.9) |
| Other doctor | 781 (62.7) | 674 (51.3) | 731 (59.2) | 724 (54.6) | 1455 (56.8) |
| Other | 37 (3.0) | 42 (3.2) | 49 (4.0) | 30 (2.3) | 79 (3.1) |
| Missing | 137 (11.0) | 217 (16.5) | 160 (12.9) | 194 (14.7) | 354 (13.9) |
| Number of units transfused, n (%) | |||||
| One | 414 (33.3) | 353 (26.8) | 355 (28.7) | 412 (31.1) | 767 (30.0) |
| Two or more | 818 (65.7) | 940 (71.5) | 864 (70.0) | 894 (67.5) | 1758 (68.7) |
| Missing | 13 (1.0) | 22 (1.7) | 16 (1.3) | 19 (1.4) | 35 (1.4) |
The health-care professional making the decision to transfuse was usually the consultant or other doctor, with this being more commonly a junior doctor postoperatively.
Appendix 2, Table 11, shows that patient-level characteristics in the follow-up audit were generally well balanced. Recruitment bias following cluster randomisation was therefore minimal, with imbalances likely to result from missing clusters. The characteristics of patients at baseline and at follow-up are similar.
Outcomes at baseline
Patient-level outcomes at baseline are provided by content and follow-on in Appendix 2, Table 12. The proportion of missing data for the primary outcome was 7%, well balanced by follow-on but less well balanced by content. Around 80% of patients had a pre- or postoperative transfusion outside guidelines: 82% of patients received a preoperative and 78% a postoperative transfusion outside guidelines. On average, 2.2 units of blood were transfused per patient by content and follow-on.
A large number of patients were excluded from the audit standards: 31% from standard 1, 91% from standard 2, 95% from standard 3, 91% from standard 4 and 6% from standard 8. Feedback given to clusters was therefore limited to specific patient groups, limiting the ability of clusters to make changes at scale. A significant group of patients could not be classified for standard 1 (28%) and standard 8 (27%). Approximately 26% of patients had a transfusion classified as not meeting standard 1, 7% had one classified as not meeting standard 2, 2% had one classified as not meeting standard 3, 6% had one classified as not meeting standard 4 and 64% had one classified as not meeting standard 8. Thus, the greatest room for improvement was for standard 8, and missing data were substantial. As expected, patient outcomes at baseline were well balanced across content and follow-on.
Outcomes at follow-up
Patient-level outcomes at follow-up are provided by enhanced content and follow-on groups in Table 3 (other outcomes are summarised in Appendix 2, Table 13). The proportion of missing data for the primary outcome was 11%, balanced across randomised groups. Approximately 73% of patients had a pre- or postoperative transfusion outside guidelines; again, the proportions were similar between randomised groups. Overall, 62% of patients receiving a preoperative transfusion and 72% receiving a postoperative transfusion received one outside guidelines.
| Variable | Content | Follow-on | Total (N = 2222) | ||
|---|---|---|---|---|---|
| Standard (N = 1224) | Enhanced (N = 998) | Standard (N = 1118) | Enhanced (N = 1104) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 198 (16.2) | 152 (15.2) | 176 (15.7) | 174 (15.8) | 350 (15.8) |
| Outside guidelines | 901 (73.6) | 726 (72.7) | 822 (73.5) | 805 (72.9) | 1627 (73.2) |
| Unclassified: ACI status unknown (Hb 70–80 g/l) | 1 (0.1) | 2 (0.2) | 3 (0.3) | 0 (0.0) | 3 (0.1) |
| Unclassified: Hb level missing | 124 (10.1) | 118 (11.8) | 117 (10.5) | 125 (11.3) | 242 (10.9) |
| Secondary outcome, mean (SD), n | |||||
| Total volume of blood transfused (units) | 2.0 (1.22), 1147 | 2.2 (1.71), 921 | 2.1 (1.62), 1052 | 2.1 (1.28), 1016 | 2.1 (1.46), 2068 |
The proportions were similar across randomised groups in postoperative transfusions, and these contributed to a greater extent to the primary outcome than preoperative transfusions. On average, 2.1 units of blood were transfused per patient, similar across randomised groups. Similar numbers of patients were excluded from audit standards at follow-up. Again, a significant group of patients could not be classified for standard 1 (22%) or standard 8 (33%). Approximately 27% of patients had a transfusion classified as not meeting standard 1, 6% had one classified as not meeting standard 2, 2% had one classified as not meeting standard 3, 5% had one classified as not meeting standard 4 and 57% had one classified as not meeting standard 8. The greatest difference observed between randomised groups was for standard 8, but the number of missing data was substantial.
Primary outcomes
Table 4 shows the primary outcome results (with the main sensitivity analysis in Appendix 2, Table 14). Across 100 imputations, the unadjusted proportion of acceptable transfusions was 18% for those allocated to standard content and to enhanced content, and the adjusted risk difference was –1% (95% CI –7% to 4%). There was no evidence of a clinically or statistically significant effect. The unadjusted proportion of acceptable transfusions was 18% for those allocated to standard or enhanced follow-on, and the adjusted risk difference was 1% (95% CI –5% to 6%), again providing no evidence of a statistically significant effect. For the follow-on intervention, a clinically important effect size of 5% (in the presence of an interaction) was not ruled out. The interaction between content and follow-on is given for information. The conclusions were unchanged regardless of the method adopted for handling the missing data. The estimates were also similar, indicating that this result is robust.
| Analysis | Unadjusted proportion acceptable | Estimated adjusted risk difference (95% CI) | Estimated adjusted odds ratio (97.5% CI) | Estimated adjusted odds ratio (95% CI) | p-value | n | |
|---|---|---|---|---|---|---|---|
| Standard | Enhanced | ||||||
| Content | 0.184 | 0.176 | –0.01 (–0.07 to 0.04) | 0.91 (0.61 to 1.36) | 0.91 (0.64 to 1.30) | 0.605 | 2222 |
| Follow-on | 0.181 | 0.180 | 0.01 (–0.05 to 0.06) | 1.05 (0.68 to 1.61) | 1.05 (0.72 to 1.52) | 0.807 | 2222 |
| Interaction | 0.184 | 0.167 | 0.05 (–0.08 to 0.13) | 1.15 (0.52 to 2.56) | 1.15 (0.57 to 2.31) | 0.696 | 2222 |
Secondary outcomes
Appendix 2, Table 17, describes the cluster-level volume of RBCs transfused (BSMS) across baseline and follow-up (and in 3-month periods). Overall, seven and six clusters, respectively, were lost to follow-up. Data were skewed; interquartile ranges (IQRs) are similar across randomised groups at baseline and follow-up. There is an indication of a steady overall reduction in blood use over this period. Appendix 2, Tables 12 and 13 summarises the patient-level volume of RBCs transfused (NCA), in each case separately for preoperative and postoperative transfusions. Imbalances are observed at baseline by randomised group, expected to be related to imbalances in missing clusters.
Appendix 2, Table 16, describes the cluster-level total number of SHOT incidents and number of relevant errors. There were no missing SHOT data, as all 135 clusters provided data, summarised across baseline and follow-up (and in 3-month intervals). Interpretation is limited because of sparse data relating to the number of relevant errors. The median number of relevant events across the baseline and follow-up periods was zero across the randomised groups. IQRs were the same across baseline and follow-up. Therefore, there is no evidence that the trial interventions increased or decreased secondary outcomes reported to BSMS, NCA or SHOT.
Appendix 2, Table 15, reports supportive outcomes.
Workstream 2a: trial 2
Methods
Study design and setting
The trial 2 methods were similar to those in trial 1. We conducted a second 2 × 2 factorial, cross-sectional, cluster-randomised controlled trial, embedded in 2016/17 NCABT, of RBC and platelet transfusions in haematology patients.
Cluster eligibility
Cluster eligibility was assessed separately for trial 2 using the same criteria as for trial 1. Where possible, cluster definitions remained the same across trials; however, in some cases, different hospital sites within clusters signed up to the audits.
Randomisation
As previously, the trial statistician undertook re-randomisation at a single point following receipt of the NCA baseline audit database. We independently randomised trusts or health boards on a 1 : 1 : 1 : 1 basis, as before balancing for trust size and regional transfusion committee, but also for the previous treatment allocation where clusters had been entered into both trials.
Interventions
The interventions remained the same across the trials, although the content of the feedback reports was tailored to the audit topic.
Monitoring intervention adherence
As before, the assessment of fidelity was based on a fidelity framework proposed by the NIH Behaviour Change Consortium. 22
Data collection
The required data and collection time points for trial 2 mirrored those in Table 1, but baseline was from July 2015 to June 2016 and follow-up was from July 2016 to June 2017.
Outcomes
Transfusion may occur for RBCs or platelets; all patients will have had one or more transfusions. The primary outcome, measured at the patient level and taken from the NCA follow-up audit, was to compare how many RBC and/or platelet transfusions were acceptable with how many transfusions were carried out outside guidelines. The clinical algorithm (see Appendix 1) was agreed by an independent panel based on clinical relevance and baseline compliance. No clinical judgement was required at a patient level to categorise transfusions.
Secondary outcomes comprised:
-
total volume of RBCs transfused (units at trust level, from BSMS summed over blood groups and clinical specialties)
-
total volume of RBCs transfused (units at patient level, from NCA)
-
total volume of platelets transfused (units at patient level, from NCA)
-
total number of incidents reported to SHOT (count at trust level, from SHOT summed over clinical specialties and events, near-misses and ‘right blood right patient’ incidents)
-
number of definitely, probably or possibly preventable incidents reported to SHOT within clinical specialties targeted by the audit (count at trust level, from SHOT summed over events, near-misses and ‘right blood right patient’ incidents).
Supportive outcomes comprised:
-
RBC transfusion (acceptable/outside guidelines)
-
platelet transfusion (acceptable/outside guidelines)
-
individual NCA audit standard met (1, 2, 3, 6 and 7; yes/no)
-
total volume of RBCs issued (units at trust level, from BSMS data)
-
total volume of RBCs transfused (units at trust level, from BSMS data).
As in trial 1, all other outcomes (i.e. intermediate NCA outcomes, number of relevant near-miss or ‘right blood right patient’ SHOT incidents, total number of unpredictable SHOT incidents, total number of possibly preventable SHOT incidents, BSMS total volume of RBCs wasted) reported are exploratory.
Sample size
As previously, we required 17–68 patients (mean 45) from 152 clusters in order to detect a minimally important reduction of 6% (to 14%) in the presence of, at most, a small antagonistic statistical interaction (i.e. main effects of 5%) with 80% power using logistic regression models, with a random-intercept for cluster, and a two-sided 2.5% significance level, for each comparison.
Statistical analysis
No interim analyses were planned or conducted. A similar analysis strategy was adopted for trial 2, except that, when adjusting for design factors, previous allocation was added.
Results
Screening and recruitment
Prior to cluster randomisation (6 July 2016), we screened 187 NHS trusts and health boards, covering the whole UK. A total of 141 (75.4%) were included in the audit, and 135 of those (95.7%) were randomised (although one was randomised in error, so 134 were entered). Reasons for exclusion were mainly ineligibility or declining to take part in the audit, so included clusters were typical of the audit as a whole. The baseline audit comprised a total of 4372 patients, 64% of target; 68 clusters were allocated to standard content, 66 were allocated to enhanced content, 67 were allocated to standard follow-on and 67 were allocated to enhanced follow-on. An average cluster size of 33 patients was observed, with a coefficient of variation of 0.5 (Figure 4).
FIGURE 4.
Trial 2: NHS trust/health board and patient CONSORT flow diagram.
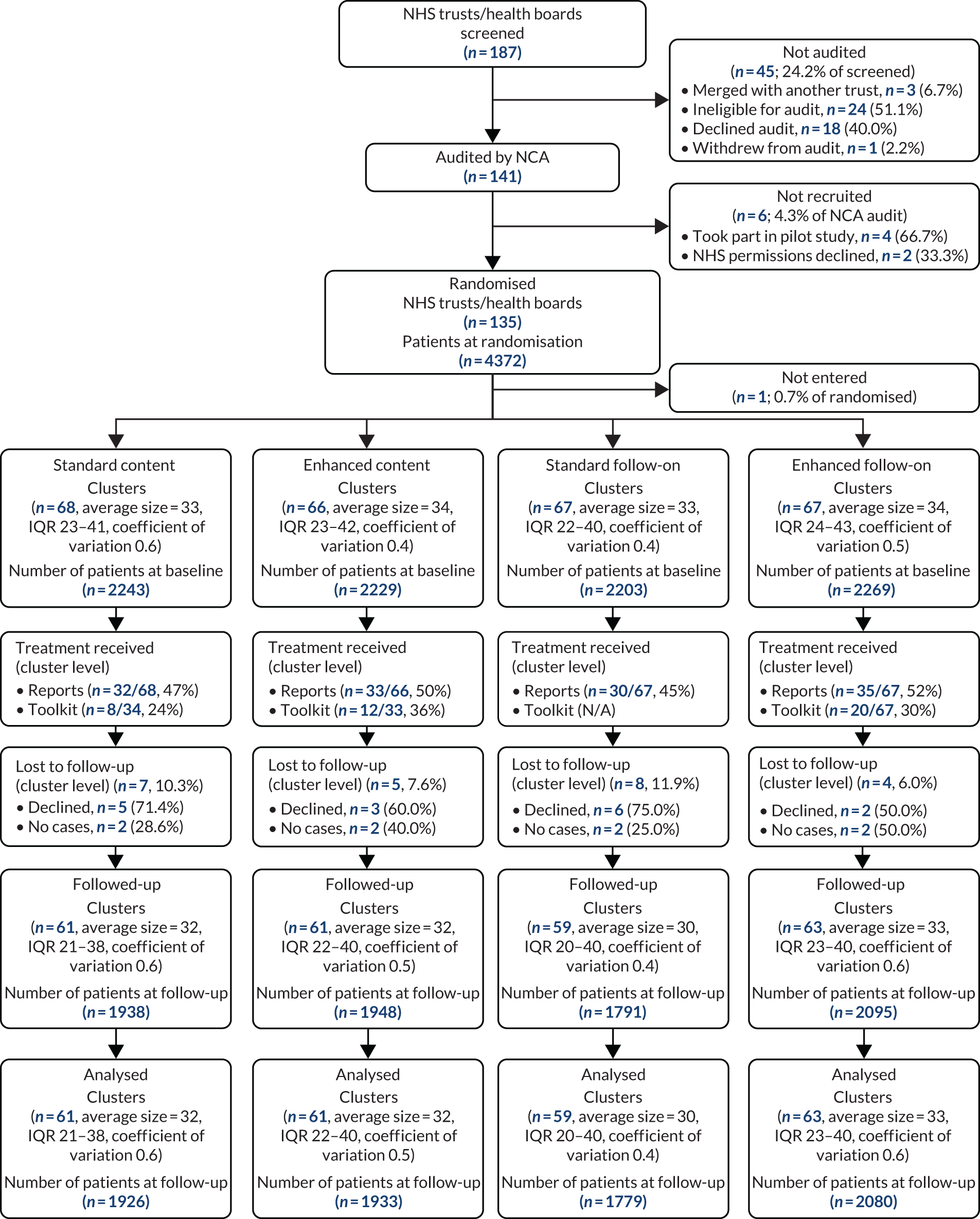
Overall, half of the clusters received the enhanced (50%) and standard reports (47%). Receipt of the toolkit was lower (30% overall), with the proportion of clusters receiving the toolkit lower among those allocated to standard content (24%) than among those allocated to enhanced content (36%). In total, 12 out of 135 clusters (9%) were lost to follow-up; similar proportions had been allocated to standard and enhanced content (10% vs. 8%) but a higher proportion had been allocated to standard follow-on than to enhanced follow-on (12% vs 6%). Overall, four clusters had no cases to audit and eight clusters declined to take part in the follow-up audit. Among the 123 clusters taking part in the follow-up audit, there were a total of 3886 audit cases. We included all 123 clusters and 3859 patients (56% of target) in our primary analyses; 27 patients were excluded because they had received a platelet transfusion only for therapeutic reasons, making them ineligible. An average cluster size of 32 patients and a coefficient of variation of 0.5 were observed at analysis.
Appendix 2, Table 18 shows the baseline patient-level characteristics. (Other baseline summaries are given in Appendix 2, Table 19.) These are generally well balanced, given that they were ascertained prior to randomisation. Overall, 1387 (31%) patients received a RBC and platelet transfusion, 2781 (63%) patients received only a RBC transfusion and 271 (6%) patients received only a platelet transfusion. For this reason, the majority categorised were RBC transfusions for medical or chronic anaemia.
Patient-level characteristics in the follow-up audit are given in Appendix 2, Table 20. These are as balanced as they were at baseline, providing no indication of recruitment bias after cluster randomisation. Characteristics of patients at baseline and follow-up are also comparable, except for the overall proportion of patients receiving a RBC transfusion undergoing a chronic transfusion programme (33% vs. 25% at baseline).
Outcomes at baseline
Patient-level outcomes at baseline are given by content and follow-on group in Appendix 2, Table 21, together with detailed definitions. The proportion of missing data for the primary outcome was 12%, balanced by content and follow-on. Around 26% of patients received a RBC or platelet transfusion outside guidelines. Overall, 11% of patients receiving a RBC transfusion and 45% receiving a platelet transfusion received one outside guidelines. On average, 2.0 units of RBCs and 1.0 units of platelets were transfused per patient across content and follow-on groups.
As in trial 1, a large number of patients were excluded from the audit standards, 6% for standard 1, 75% for standard 2, 99% for standard 3, 90% for standard 6 and 71% for standard 7, owing to the specific subset of patients considered in each standard. As such, the feedback given to clusters was focused on specific patient groups, thereby limiting the ability of clusters to make changes that would apply to all patients. Few patients could not be classified (0% for standards 1, 3 and 7; 2% for standard 2; and 7% for standard 6). Of those eligible, 6% of patients had a transfusion classified as not meeting standard 1, 73% as not meeting standard 2, 47% as not meeting standard 3, 42% as not meeting standard 6 and 38% as not meeting standard 7. Thus, the greatest improvement was required in standards 2, 3, 6 and 7, which focused on specific patient groups. As expected, patient outcomes at baseline were well balanced across content and follow-on groups.
Outcomes at follow-up
Patient-level outcomes at follow-up are given by content and follow-on in Table 5 (other outcome summaries are given in Appendix 2). The proportion of missing data for the primary outcome was 9%, balanced by randomised groups. About 25% of patients received a RBC or platelet transfusion outside guidelines, and the proportions were similar between randomised groups. Overall, 11% of patients receiving a RBC transfusion and 45% receiving a platelet transfusion received one outside guidelines. Again, proportions were similar between randomised groups. On average, 2.0 units of RBCs and 1.0 units of platelets were transfused per patient, the same across randomised groups.
| Variable | Content | Follow-on | Total (N = 3859) | ||
|---|---|---|---|---|---|
| Standard (N = 1926) | Enhanced (N = 1933) | Standard (N = 1779) | Enhanced (N = 2080) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 1308 (67.9) | 1226 (63.4) | 1196 (67.2) | 1338 (64.3) | 2534 (65.7) |
| Outside guidelines | 457 (23.7) | 507 (26.2) | 433 (24.3) | 531 (25.5) | 964 (25.0) |
| Unclassified | 161 (8.4) | 200 (10.3) | 150 (8.4) | 211 (10.1) | 361 (9.4) |
| Secondary outcome | |||||
| RBC transfusions, n | 1815 | 1832 | 1674 | 1973 | 3647 |
| Volume transfused, median (IQR), n | 2.0 (1.0–2.0), 1813 | 2.0 (1.0–2.0), 1829 | 2.0 (1.0–2.0), 1671 | 2.0 (1.0–2.0), 1971 | 2.0 (1.0–2.0), 3642 |
| Platelet transfusions, n | 729 | 717 | 633 | 813 | 1446 |
| Volume transfused, median (IQR), n | 1.0 (1.0–1.0), 716 | 1.0 (1.0–1.0), 705 | 1.0 (1.0–1.0), 626 | 1.0 (1.0–1.0), 795 | 1.0 (1.0–1.0), 1421 |
Similar numbers of patients were excluded from each audit standard at follow-up. Again, only a few patients could not be classified, with the exception of standard 3, for which 12% could not be classified. Of those eligible, 6% of patients had a transfusion classified as not meeting standard 1, 72% as not meeting standard 2, 21% as not meeting standard 3, 42% as not meeting standard 6 and 36% as not meeting standard 7. Differences between enhanced and standard content were indicated for standards 2, 3, 6 and 7, favouring enhanced content for standards 3 and 7 and favouring standard content for standards 2 and 6. Differences between enhanced and standard follow-on were minimal across all the standards.
Primary outcomes
Table 6 shows primary outcome results (sensitivity analyses are given in Appendix 2, Table 23). Across 100 imputations, the unadjusted proportion of acceptable transfusions was 74% for those allocated to standard content and 71% for those allocated to enhanced content; the adjusted risk difference was –4% (95% CI –9% to 2%). There was no evidence of a clinically or statistically significant effect. The unadjusted proportion of acceptable transfusions was 74% for those allocated to standard follow-on and 72% for those allocated to enhanced follow-on; the adjusted risk difference was –1% (95% CI –6% to 5%), again indicating no evidence of a statistically or clinically important effect. The interaction between content and follow-on is again given for information. The conclusions were unchanged regardless of the method adopted for handling the missing data. The estimates are also similar, indicating that this result is robust.
| Analysis | Unadjusted proportion acceptable standard | Unadjusted proportion acceptable enhanced | Estimated adjusted risk difference (95% CI) | Estimated adjusted odds ratio (97.5% CI) | Estimated adjusted odds ratio (95% CI) | p-value | n |
|---|---|---|---|---|---|---|---|
| Content | 0.744 | 0.714 | –0.04 (–0.10 to 0.01) | 0.81 (0.56 to 1.12) | 0.81 (0.60 to 1.08) | 0.148 | 3859 |
| Follow-on | 0.739 | 0.721 | –0.01 (–0.06 to 0.05) | 0.96 (0.67 to 1.38) | 0.96 (0.71 to 1.32) | 0.823 | 3859 |
| Interaction | 0.737 | 0.707 | 0.03 (–0.08 to 0.14) | 1.22 (0.60 to 2.48) | 1.22 (0.66 to 2.27) | 0.522 | 3859 |
Secondary outcomes
Cluster-level volume of RBCs transfused (BSMS) is summarised descriptively across baseline and follow-up (and in 3-month periods) in Appendix 2, Table 26. Overall, three clusters were lost to follow-up. Data were again skewed; IQRs are again similar across randomised groups at baseline and follow-up. Patient-level volume of blood components transfused (NCA) is summarised descriptively in Appendix 2, Tables 21 and 22, in each case separately for RBC and platelet transfusions. The conclusions are consistent. Cluster-level total number of SHOT incidents and number of relevant errors are summarised descriptively in Appendix 2, Table 25. There were no missing SHOT data, as all 134 clusters provided data, summarised across baseline and follow-up (and in 3-month intervals). Again, sparse data relating to the number of relevant errors limit the interpretation of these. The median number of relevant events across randomised groups was similar at baseline and follow-up; IQRs were also similar. As in trial 1, there is no evidence that the trial interventions changed secondary outcomes reported to BSMS, NCA or SHOT.
Appendix 2, Table 24 reports supportive outcomes.
Limitations
Four main factors affected the interpretation of the cluster-randomised trials. First, the number of clusters participating in each audit and in the trials, as well as the number of patient records audited in each cluster, was smaller than projected. This compromised statistical power, increasing uncertainty around our estimates of intervention effects. However, the results appeared sufficiently consistent across the trials, the two interventions, and the range of primary and secondary outcomes for us to conclude that it is highly unlikely that the enhanced interventions had any important effects. Second, randomised clusters lost to follow-up were not included in analyses. Further sensitivity analyses are required to explore the impact of this. Third, the audit data contributing to trial outcome measures were more complex and required more review and cleaning than anticipated. Fourth, the audit standards that required most improvement were relevant to only a subset of patients included in the audits. This limited the ability of the trial to detect change in practice at scale, as it diluted the underlying effects of the interventions.
Workstream 2b: cost-effectiveness analysis
Aim
The economic modelling evaluated the costs and benefits of the two AFFINITIE A&F interventions in two trials, and aimed to assess the interventions’ cost-effectiveness.
Methods
Design overview and model
The analysis was conducted using decision-analytic modelling from the perspective of the NHS. For each of trials 1 and 2 we compared the costs and the outcomes of the ‘enhanced content’ with ‘usual content’ arms, and the ‘enhanced follow-on support’ with ‘usual follow-on support’ arms (Figure 5). We explored uncertainty around the parameters used in the model using sensitivity analysis.
FIGURE 5.
Cost-effectiveness analysis model for trials 1 and 2.
The primary outcome of the trials was the proportion of transfusions given that were acceptable. For ease of understanding the incremental cost-effectiveness ratios (ICERs) presented in this analysis, we multiplied those proportions by 100 so that they were percentages. Therefore, the primary outcome of this analysis was the percentage of acceptable transfusions. Secondary outcomes comprised the volume of blood transfused and the number of SHOT-reportable incidents. The time horizon for this analysis was 1 year, during which two rounds of audits took place for each trial. We applied no discounting.
Unit of analysis
The unit of analysis for this evaluation was per site, reflecting the fact that the intervention was delivered at secondary care sites, and that many of the cost data available were for local, site-level costs. This represented a change from the original protocol, in which the unit of analysis was ‘per patient.’
Analysis overview and changes to protocol
The analysis presented follows the original proposal, with the following amendments:
-
The unit of analysis was originally ‘per patient’ but was changed to ‘per site’, as the costs were collected at trust and whole intervention level, and the interventions were delivered at the site level.
-
The number of transfusion-related adverse events used in the economic analysis was taken from the results of the statistical analysis and was not modelled in the long-term, reflecting the rest of the analyses’ time horizon.
-
The analysis was amended to undertake four two-arm comparisons, rather than two four-arm comparisons, to be appropriate to the overall study design.
-
The protocol intended quality-adjusted life-years (QALYs) to be estimated from SHOT events. However, the number of these events was small and there was no significant difference in SHOT events between the comparison groups, meaning that a cost–utility analysis would not yield meaningful results.
Costing exercise methodology
The costs of the intervention were collected from a combination of top-down (gross-costing) and bottom-up (microcosting) methods, depending on the type and the quality of data available. An extensive costing exercise was undertaken to estimate the resources required to deliver the A&F interventions and the standard practice audits. The resource components included in this exercise were collecting the audit data, developing and delivering the audit reports, delivering the follow-on-support programmes, additional NHS activity in response to receiving the audit reports and the volume of blood transfused. The costs associated with the volume of blood transfused were far higher than the other costs combined. There was a large degree of uncertainty around these cost estimates, particularly around the amount of time NHS staff spent collecting and inputting audit data and using the online toolkit, and the volume of blood transfused per site. This uncertainty is explored in the sensitivity analyses. The costing exercise methodology and results are detailed in Report Supplementary Material 18.
Unit costs
The cost used for purchasing a unit of blood components was £128.99 and £185.86 for RBCs and platelets, respectively. 23 We applied a cost of £51.32 for transfusing 1 unit of blood components. This was obtained using the value given for subsequent units of blood transfused in the costing statement of the National Institute for Health and Care Excellence (NICE) guidelines for blood transfusion and management,24 and inflating the difference for 2017/18.
Therefore, the unit cost of one unit of RBCs and platelets was £175.78 and £229.51, respectively. Unit costs of staff time were drawn from the NHS pay scale 2017/18,25 the British Medical Association pay scales for junior doctors 2017/18,26 and the British Medical Association pay scales for consultants 2017/18. 27 Non-clinical staff costs were drawn from the health-care or research organisation employing the relevant staff member (i.e. NHSBT; University of Leeds; or City, University of London).
Outcome parameters
Outcome data were obtained from the outputs of the trial analyses (see Tables 4 and 6). Where necessary, these outcomes were converted from mean-per-cluster to mean-per-site to conform to the unit of analysis for this study. Table 7 presents the parameters used in the analysis for the primary and secondary outcomes for trial 1, and Appendix 3, Table 28, describes those for trial 2.
| Percentage of transfusions acceptable | Units of blood transfused per arm (SD) | Mean number of units per site (SD) | Number of SHOT events (SD) | Mean per site (SD) | |
|---|---|---|---|---|---|
| Standard content | 18.4 | 516,499 (371,291) | 2792 (2007) | 1193 (1441) | 6.5 (7.8) |
| Enhanced content | 17.6 | 658,951 (473,650) | 3562 (2560) | 1159 (967) | 6.3 (5.2) |
| Standard follow-on support | 18.1 | 531,618 (359,462) | 2874 (1943) | 1059 (938) | 5.7 (5.1) |
| Enhanced follow-on support | 18.0 | 589,301 (450,541) | 3185 (2435) | 1293 (1466) | 7.0 (7.9) |
Cost-effectiveness analysis methodology
We calculated ICERs to measure the cost-effectiveness for each comparative analysis for trials 1 and 2. The ICER is interpreted as the additional cost required to produce one unit of benefit. For the primary outcome, benefit is defined as one additional percentage of blood transfused acceptably. For SHOT it is one fewer SHOT-reportable event. We treated the volume of blood used as a resource consumed and not as a health consequence, and thus included it only on the cost side of the ICER calculations.
The ICER calculation shown in Appendix 3, Tables 27 and 28, demonstrates how the different cost components were included in the calculation of the ICER for the primary outcome.
Sensitivity analysis methodology
To explore the uncertainty around the parameters used in our model, we conducted probabilistic sensitivity analysis (PSA) and deterministic sensitivity analysis (DSA). Each parameter in the DSA was varied by 20% above and below the mean value. For the PSA all cost parameters were characterised using a gamma distribution, as was the volume of blood transfused and the number of SHOT events. The proportion of acceptable transfusions was characterised using a beta distribution.
We ran 1000 iterations for each PSA comparison and presented the results in terms of mean incremental difference and associated uncertainty intervals (UIs) on a cost-effectiveness plane. A cost-effectiveness acceptability curve (CEAC) was plotted for each analysis. We found that PSA iterations fell into all four quadrants of the cost-effectiveness plane. To calculate the ICER relative to willingness-to-pay (WTP) thresholds for the CEAC, we took the following approach:
-
All iterations in the north-west quadrant were never considered to be cost-effective.
-
All iterations in the south-east quadrant were always considered to be cost-effective.
-
Iterations in the north-east quadrant had to fall below the WTP threshold to be considered cost-effective.
-
Iterations in the south-west quadrant had to be above the WTP threshold to be considered cost-effective.
Budget impact and cost-neutral analyses
We calculated the effect of each intervention on all 185 participating sites in trial 1 and 194 sites in trial 2 to estimate the effect on the NHS budget at a national level. We then adopted a cost-neutral framework by asking ‘How many units of blood would need to be prevented from being transfused in order for the interventions to be cost neutral (i.e. to break even)?’. For this analysis we evaluated the cost of each intervention in terms of the equivalent cost in units of RBCs. We did this by calculating the incremental costs of each pair of interventions (enhanced minus standard) for each trial, and dividing the result by the cost of a unit of RBCs. We used sensitivity analysis to explore the impact that the intervention costs and unit costs of RBCs had on the results.
Results
Intervention costs: budget impact analysis
Table 8 presents the cost of each arm for both trials. The highest costs across all arms were those of the blood transfused. The lowest costs were those of the feedback interventions.
| Standard | Enhanced | Difference (i.e. additional cost of enhanced) | ||||
|---|---|---|---|---|---|---|
| Cost for all 185 sites (£) | Cost per site (£) | Cost for all 185 sites (£) | Cost per site (£) | All sites (£) | Per site (£) | |
| Content | ||||||
| Audit data collection | 93,841 | 507 | 93,841 | 507 | 0 | 0 |
| Feedback interventions | 22,739 | 123 | 66,604 | 360 | 43,864 | 237 |
| Additional NHS activity | 228,666 | 1236 | 225,228 | 1217 | –3438 | –19 |
| Blood transfused | 90,789,344 | 490,753 | 115,829,314 | 626,104 | 25,039,970 | 135,351 |
| Total | 91,134,590 | 492,619 | 116,214,986 | 628,165 | 25,080,396 | 135,570 |
| Follow-on | ||||||
| Audit data collection | 93,841 | 507 | 93,841 | 507 | 0 | 0 |
| Feedback interventions | 22,739 | 123 | 28,721 | 155 | 5982 | 32 |
| Additional NHS activity | 228,311 | 1234 | 225,625 | 1220 | –2686 | –15 |
| Blood transfused | 93,446,854 | 505,118 | 103,586,378 | 559,926 | 10,139,524 | 54,808 |
| Total | 93,791,745 | 506,982 | 103,934,565 | 561,808 | 10,142,820 | 54,826 |
Excluding the cost of blood transfusions, in trial 1 the incremental cost of the enhanced compared with the standard content intervention was £219 per site, and the incremental cost of the enhanced compared with the standard follow-on support intervention was £18 per site. For trial 2 these figures were £248 and –£198, respectively. Owing to the cost of additional NHS activity, enhanced follow-on support cost less per site than standard support.
Cost-effectiveness analysis results: primary outcome
The cost per site of standard content and enhanced feedback content was £492,619 and £628,189, respectively. Enhanced feedback content cost £135,570 more per site than standard content, and was associated with a 0.8% decrease in acceptable transfusions. Therefore, the base-case ICER was –£169,462 and the enhanced intervention was dominated by the standard intervention.
The cost per site of standard follow-on support and enhanced follow-on support was £506,982 and £561,826, respectively. Enhanced follow-on support cost £54,826 per site more than standard follow-on support, and was associated with a 0.1% decrease in acceptable transfusions. Therefore, the base-case ICER was –£548,261 and the enhanced intervention was dominated by the standard intervention.
The cost per site of the standard content and enhanced feedback content was £675,173 and £689,873, respectively. Enhanced content cost £14,700 more per site than standard content, and was associated with a 3.0% decrease in acceptable transfusions. Therefore, the base-case ICER was –£4900 and the enhanced intervention was dominated by the standard intervention.
The cost per site of standard follow-on support and enhanced follow-on support was £582,805 and £617,084, respectively. Enhanced follow-on support cost £34,278 per site more than standard follow-on support, and was associated with a 1.8% decrease in acceptable transfusions. Therefore, the base-case ICER was –£19,044 and the enhanced intervention was dominated by the standard intervention.
Hence, in every case, the enhanced intervention was dominated by the standard intervention. This was also the case if we included only the cost of the interventions and related activity (i.e. not the cost of blood transfusions).
Cost-effectiveness analysis results: secondary outcome (number of SHOT events)
Enhanced feedback content saw a reduction in SHOT events of 0.2 per site compared with standard content, producing a base-case ICER of £735,927 per one-unit reduction in SHOT events. Enhanced follow-on support saw an increase in SHOT events of 1.3 per site compared with standard follow-on support, producing a base-case ICER of –£43,455 and resulting in the enhanced intervention being dominated by the standard intervention.
The results for trial 2 can be found in Report Supplementary Material 19.
Probabilistic sensitivity analysis: primary outcome
For each of the following analyses there is considerable uncertainty in the estimates produced, indicated by the wide uncertainty intervals, which include zero.
Trial 1: percentage of transfusions acceptable (enhanced versus standard content)
The PSA results suggest that the mean incremental cost of the enhanced versus standard intervention was £129,449 per site (95% UI –£1,092,049 to £1,350,946 per site). The mean incremental change in percentage of acceptable transfusions was –0.81% (95% UI –3.1% to 1.4%). This indicates that the enhanced intervention was more costly and less effective than the standard intervention.
Figure 6 shows the simulated outputs shown on a cost-effectiveness plane (CEP).
FIGURE 6.
Simulated outputs on the cost-effectiveness plane for trial 1 enhanced vs. standard content for the primary outcome. Each blue diamond represents one individual simulated output of the model.
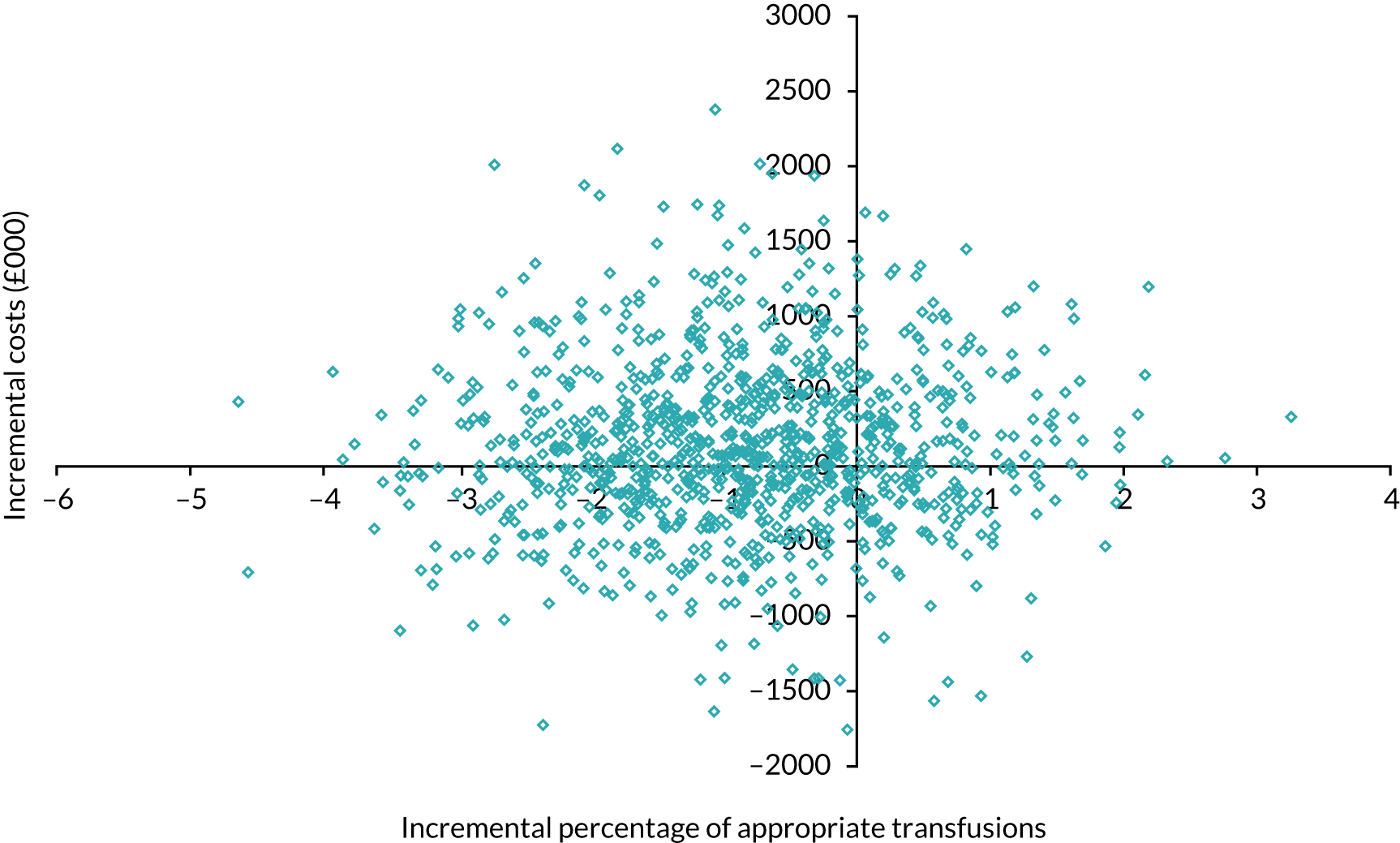
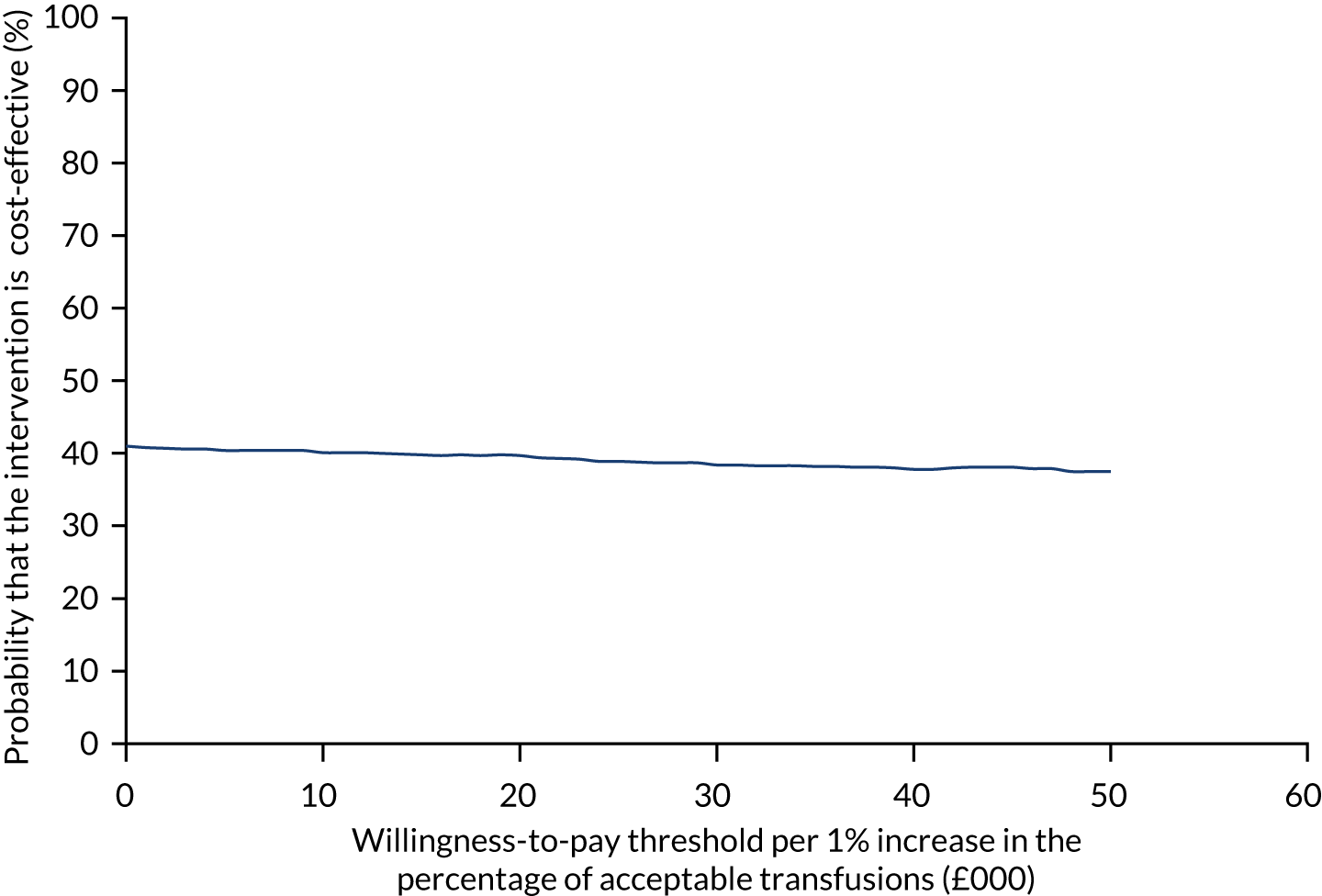
Figure 7 shows the probability of the enhanced intervention being cost-effective at increasing WTP thresholds. The slight downwards-trending CEAC is due to more iterations falling in the south-west quadrant than in the northeast quadrant (32% vs. 14%, respectively), and as the WTP threshold increases more iterations in the south-west are excluded from being cost-effective than iterations in the north-east are included as being cost-effective.
FIGURE 7.
The CEAC for trial 1 enhanced vs. standard content for the primary outcome. The navy line represents the probability that the intervention is cost-effective at ascending WTP thresholds.
Trial 1: percentage of transfusions acceptable (enhanced versus standard follow-on support)
The PSA results suggest that the mean incremental cost of the enhanced versus standard intervention was £64,319 per site (95% UI –£1,003,034 to £1,131,673 per site). The mean incremental change in percentage of acceptable transfusions was –0.11% (95% UI –2.38% to 2.16%). Therefore, the enhanced intervention was more costly and less effective than the standard intervention.
Figure 8 shows the simulated outputs on a cost-effectiveness plane (CEP).
FIGURE 8.
A CEP for enhanced vs. standard follow-on support for percentage of acceptable transfusions.
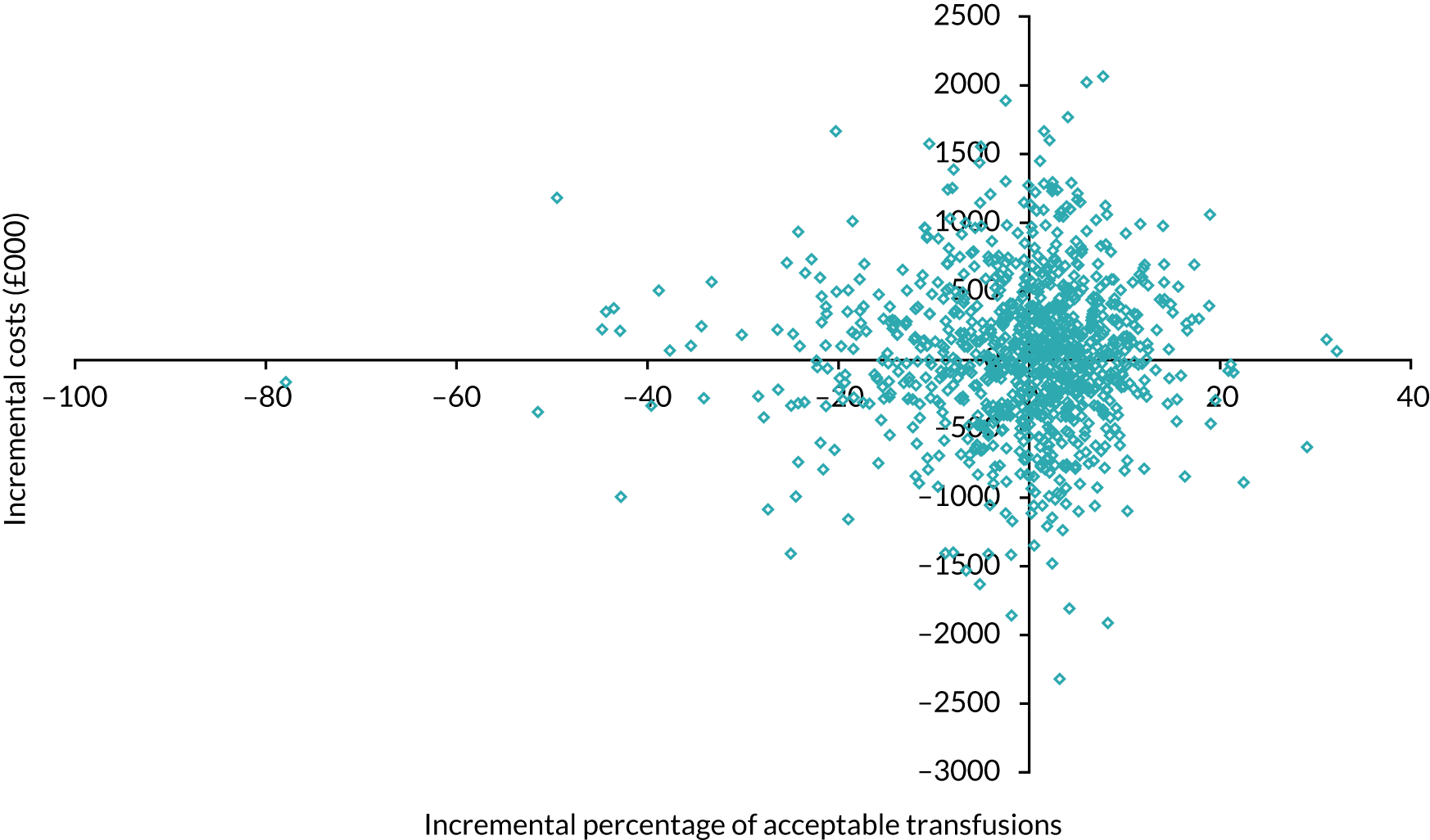
Figure 9 shows the CEAC holding fairly constant at 45% probable cost-effectiveness. This is because the number of iterations in the south-west quadrant that are found to not be cost-effective is roughly similar to the number in the north-east quadrant that are found to be cost-effective and this remains the case as the WTP threshold increases. See Appendix 3 for further results tables on trial 2.
FIGURE 9.
The CEAC for enhanced vs. standard follow-on support for percentage of acceptable transfusions.
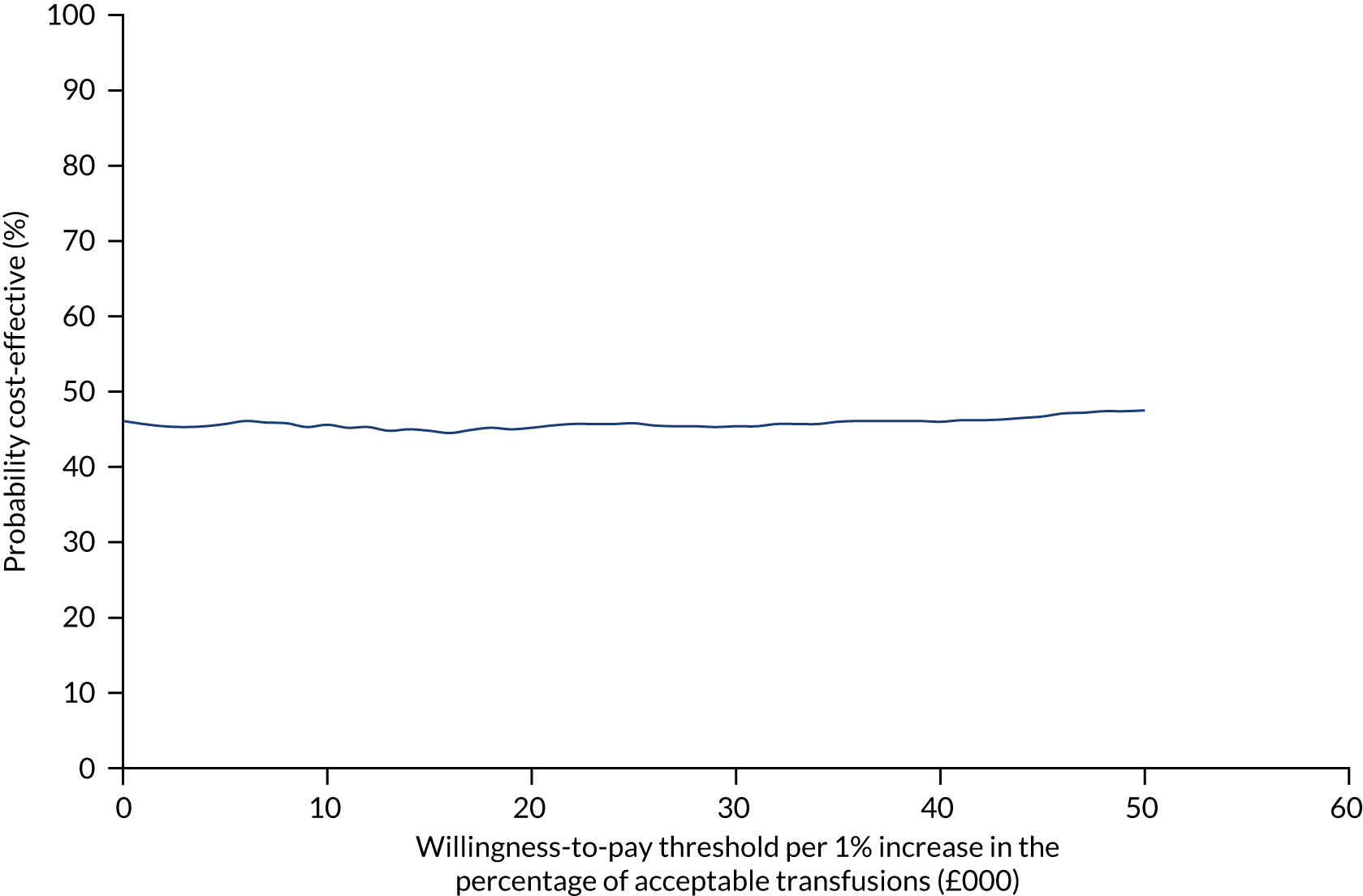
Deterministic sensitivity analysis results
Varying all of the intervention components had a negligible effect on the model, with the exception of the cost of blood transfused. Varying the cost per site of RBCs and platelets had a large effect on the model, as did varying the percentage of appropriate transfusions and the number of SHOT events. The detailed results of the DSA are presented in Report Supplementary Material 19.
Cost-neutral analysis
For trial 1, the mean incremental cost of the enhanced content intervention compared with the standard content intervention was £219 per site and of enhanced versus standard follow-on support was £18 per site. Hence, the interventions would need to reduce the volume of unacceptable blood transfusions by 1.2 and 0.1 units of RBCs per site, respectively, to be cost neutral.
In trial 2, the mean incremental cost of the enhanced content intervention compared with the standard content intervention was £248 per site and the mean incremental cost of enhanced compared with standard follow-on support was –£198 per site. Hence, the interventions would need to reduce the volume of unacceptable blood transfusions by 1.4 and 0.0 units of RBCs per site, respectively, to be cost neutral. The incremental costs and equivalent units of RBCs are presented in Table 9 (comparable results for trial 2 are shown in Appendix 3, Table 29).
| Cost per site (£) | Units of RBCs equivalent needed to be cost neutral | Units if RBCs cost 20% less | Units if RBCs cost 20% more | |
|---|---|---|---|---|
| Enhanced vs. standard content | ||||
| Incremental mean | 219 | 1.2 | 1.6 | 1.0 |
| 95% UI upper bound | 630 | 3.6 | 4.5 | 3.0 |
| 95% UI lower bound | –193 | –1.1 | –1.4 | –0.9 |
| Enhanced vs. standard follow-on support | ||||
| Incremental mean | 18 | 0.1 | 0.1 | 0.1 |
| 95% UI upper bound | 66 | 0.4 | 0.5 | 0.3 |
| 95% UI lower bound | –30 | –0.2 | –0.2 | –0.1 |
Limitations
The economic modelling was limited by missing data around costs and also did not include costs of adverse events reported to the national haemovigilance system (SHOT). We also used site rather than patient as the unit of analysis to approximate costs and benefits at NHS trust or health board level; we judged that this would best inform national and local decision-making on the relative value of the interventions. The analysis time horizon was very short. No significant difference was found in the number of SHOT events, and the number of events was small, precluding a meaningful cost–utility analysis. Had the intervention been successful in this regard, a cost-per-QALY analysis would have been a valuable method of estimating the effect of serious harm caused by unnecessary blood transfusions on patient health outcomes. Data on additional activity that were collected by means questionnaires sent to NHS staff suffered from being poor quality and a large number of data were missing. The cost of additional activity was much higher than the costs associated with auditing and feeding back; however, the missing cost data had little impact on the model overall, as the cost of the volume of blood transfused dominated all other costs.
Workstream 3: process evaluation
Background
The two trials (see Workstream 2) identified no significant differences between either of the enhanced feedback interventions and standard practice. Process evaluations aim to understand how interventions work in practice by exploring their implementation (i.e. fidelity, dose and reach), their mechanisms of impact (i.e. participant responses to and interactions with interventions) and the contextual factors associated with variation in outcomes. 28,29 The main process variable of interest in the AFFINITIE process evaluations was fidelity,22 including the fidelity dimensions of delivery (i.e. whether or not the intervention was delivered by providers as intended), receipt (i.e. whether or not participants initially understood, recalled and engaged with the intervention) and enactment (i.e. whether or not recipients enacted target behaviours and applied the intervention as intended in their clinical practice). 30
The pragmatic trials had been embedded in routine quality improvement practice and, therefore, a wide range of potential contextual factors may also have influenced implementation, engagement and outcomes, beyond the interventions themselves. 29,31
Aims
Specific research questions included:
-
To what extent were the feedback interventions delivered by providers as designed and intended? (fidelity of delivery)
-
To what extent were the feedback interventions received, understood, and engaged with as intended by transfusion clinical staff in UK hospitals? (fidelity of receipt)
-
To what extent was change implemented in the light of feedback? (enactment)
-
To what extent did contextual factors external to the interventions influence clinical staff’s responses to feedback? (context).
Methods
Design
The full process evaluation protocol is reported elsewhere. 32 In line with process evaluation guidance,29 we used mixed methods, balancing in-depth qualitative data collection in a subsample of participating clusters with quantitative data collection across all participating clusters. We selected which aspects of fidelity to investigate (delivery, receipt, enactment), and our methodological approach to assessing these, based on published fidelity frameworks and guidance. 22,30 Researchers who analysed the process evaluation data were blind to trial findings.
Fidelity of delivery
We defined fidelity of delivery in terms of the extent to which the enhanced interventions (content and follow-on) were delivered as designed and planned. This was assessed using:
-
Upload checks. Materials for the enhanced and standard interventions (i.e. reports, links to the toolkit) were electronically delivered to sites by the NCA team, who first uploaded materials to the NCA website and then e-mailed listed contacts at each site to notify them that feedback materials were available for download. Staff at sites then logged in to the NCA website to access their site-specific feedback. At the upload stage, for all participating sites, we checked that the correct combination of feedback materials had been uploaded/delivered in accordance with each site’s allocation to trial arm. Any errors were corrected at this stage.
-
Content analyses of the final versions of the enhanced reports, web-based toolkit and delivered telephone support sessions:
-
Enhanced content. We coded the enhanced feedback reports produced by the NCA enhanced writing group for the presence/absence of the six recommended theory- and evidence-based enhancements (see Workstream 1 Intervention development and piloting and Report Supplementary Material 10) and calculated the percentage that were present.
-
Enhanced follow-on. We coded the final version of the toolkit using BCT taxonomy v1,7 and calculated how many (%) of 17 intended BCTs (see Report Supplementary Material 13) were present. This was to check that no intended components/BCTs were lost when computer scientists translated the toolkit from paper to web-based delivery. 33
-
Telephone support component of enhanced follow-on. We logged and calculated how many (%) sites in each trial arm randomised to enhanced follow-on received at least one initial telephone support call. We audio-recorded all telephone support sessions. A subsample of sessions (≈ 10%; 12 total; six per trial, two for each of the three facilitators delivering support) were transcribed and coded into component BCTs using taxonomy v1. 7 The telephone support manual specified four BCTs to be delivered during all initial telephone support sessions (prompts/cues, credible source, social support practical, and information about consequences). We calculated the percentage of these delivered in each session.
-
In line with published guidance, < 50% of delivery of intended intervention components was classed as ‘low’ fidelity, 51–79% was classed as ‘medium’ fidelity and 80–100% was classed as ‘high’ fidelity. 30
Receipt and enactment
We defined receipt30 as the extent to which hospital staff receiving the feedback interventions initially engaged with the intervention (i.e. downloaded the feedback reports, read them, logged in to the online toolkit and completed the tools), and understood and remembered the interventions and their content. Enactment30 was defined in terms of the extent to which intervention recipients engaged in four behaviours targeted by the feedback interventions: (1) disseminating feedback reports to colleagues, (2) setting localised goals, (3) developing action plans and (4) re-monitoring performance locally.
Receipt and enactment were assessed both quantitatively and qualitatively.
Quantitative assessment
Web analytics data were used to assess initial receipt. The NCA web page on which interventions were delivered was programmed to record the number of times each feedback report (enhanced and standard) was downloaded throughout the intervention period (8 months). For enhanced follow-on, the toolkit was programmed to record usage patterns (i.e. number of logins, page views, adding/deleting characters). Data were collected for all clusters participating in each trial (trial 1, n = 135; trial 2, n = 134). For clusters with multiple sites, site-level data were aggregated to cluster level. Web analytics data were summarised using descriptive statistics and compared across interventions and trials.
Qualitative assessment
In-depth semistructured qualitative interviews were conducted with 35 participants from 21 clusters in trial 1 and with 20 participants from 14 clusters in trial 2. Participants were hospital staff in the hospital transfusion team and/or the clinical specialty being audited in each trial (surgery and haematology; see Appendix 4). Participants were recruited via a study information sheet that was sent to the NCA listed contact at each site, who was asked to forward the invitation to potentially eligible staff. Interviews were conducted by trained researchers over the telephone approximately 6 months after intervention delivery. These interviews were semistructured and included questions to explore how hospitals had responded to feedback, particularly extent of receipt (i.e. whether or not the participant recalled receiving the intervention materials, how much of the feedback materials they read and ease of understanding), enactment of the aforementioned target behaviours, and factors facilitating and hindering this. The interview topic guide is available in Report Supplementary Material 20. Interviews were transcribed, anonymised and analysed using inductive thematic synthesis. 34 Themes were compared across interventions, trial arms and trials. Analysis was primarily conducted by a researcher not involved in designing and delivering the enhanced interventions. However, interpretation and proposed themes were discussed with the process evaluation research team, some of whom had been involved in the intervention design.
Context
The interview topic guide (see Report Supplementary Material 20) included questions to explore contextual influences on responses to feedback. These included items related to the inner hospital setting35 (e.g. support and engagement from colleagues, competing priorities, available resources, required skills) as well as settings35 external to the hospital (e.g. national initiatives, policies). These data were also analysed using thematic synthesis.
Results
Delivery
Overall, fidelity of delivery was high for both enhanced interventions. Upload checks confirmed that 100% of clusters in each trial received the correct combination of enhanced/standard feedback materials according to random allocation.
Enhanced content
For enhanced content, 100% of the intended enhancements were present in at least one enhanced report in both trial 1 and trial 2 (see Report Supplementary Material 21). This provided evidence that it was feasible to deliver, with good fidelity, the proposed enhancements to the design and content of feedback.
Enhanced follow-on
For enhanced follow-on, 100% of the 17 intended BCTs were identified in the final version of the web-based toolkit (see Report Supplementary Material 13). This version was used in both trials. An initial telephone support call was delivered to 89% of clusters randomised to receive enhanced follow-on in trial 1 (n = 71), and to 90% of clusters in trial 2 (n = 77). Transcripts of trial 1 telephone support sessions contained on average 96% of manual-specified BCTs, whereas trial 2 sessions contained on average 86% of manual-specified BCTs (see Report Supplementary Material 22).
Receipt and enactment
Overall, initial intervention receipt was high for enhanced content, moderate for standard content and follow-on and low for enhanced follow-on. Enactment for all interventions was moderate.
The summary of findings below focuses on trial 1, as there was little difference in receipt, enactment and contextual influences between the trials. Any differences are described narratively.
Web analytics
In trial 1, most clusters downloaded the enhanced feedback reports (summary key and full findings versions) at least once (Figure 10) [57 (82.6%) clusters receiving enhanced content and 52 clusters (78.8%) receiving standard feedback]. This percentage difference was not statistically significant (χ2(1) = 0.317; p = 0.574).
FIGURE 10.
Percentages of clusters downloading enhanced and standard feedback reports in trial 1.
In trial 1, most clusters (59; 86.8%) logged in to the toolkit at least once (see Figure 3). This was lower in trial 2 (49; 73.1%). In trial 1, the highest number of logins occurred in months 1 and 2, with the number subsequently decreasing over the intervention period (Figure 11), except in month 6, when there was a spike in logins that coincided with data collection for interviews.
FIGURE 11.
Number of logins in to the enhanced follow-on toolkit in trial 1.
Almost all toolkit enactment metrics (page views, downloads, items edited/added/deleted) had median scores of 0, indicating low engagement with the toolkit. Metrics with median scores of ≤ 1 included page views for the introduction page (median = 1), dissemination cascade (0.5) and selecting standards tool (0.5). Full descriptive statistics on all enactment metrics across both trials are available in Report Supplementary Material 23.
Interviews
Themes and supporting quotations related to receipt, enactment and context across interventions are presented in Report Supplementary Material 24–26 for trial 1 and Report Supplementary Material 27 for trial 2.
Receipt
For enhanced content, receipt was high: most participants interviewed in trial 1 recalled receiving feedback about their performance related to the elective surgery audit. Participants reported that the enhanced reports were clear, well written, comprehensible and structured in a logical order, with helpful visual representations of performance data that enabled them to readily identify their hospital’s performance. Many participants expressed a preference for the enhanced reports over standard reports. Standard content receipt was mixed: all participants in the standard content arms recalled receiving feedback reports from this audit. However, many participants reported having great difficulties with reading and interpreting feedback from standard reports. Although not all participants experienced such problems, some reported receiving summary versions produced by their hospital’s transfusion practitioner. Although the recommendations for change in both standard and enhanced reports were considered clear, some felt that the recommendations were unrealistic, for example those around the use of cell salvage machines when there was a lack of available equipment and trained clinicians (see Report Supplementary Material 24).
By contrast, receipt of enhanced follow-on was low: only six participants recalled receiving the toolkit. Many did not comment further on their understanding of and engagement with the intervention. Those who did said that they required support to use the toolkit as they struggled to use it otherwise (see Report Supplementary Material 24); however, no participants called the telephone support line that was offered as part of enhanced follow-on.
Enactment
In both trials, all participants reported enacting key behaviours targeted by the interventions, with minimal variation across trial arms and interventions. All participants shared feedback materials with colleagues, typically at hospital transfusion committee meetings. There were concerns over feedback not being disseminated to front-line staff (e.g. junior doctors). Shorter summary enhanced reports were more likely to be disseminated widely. All participants reported conducting gap analyses to set localised goals based on where their performance was poorest relative to audit standards and other hospitals. Action plans were in turn developed by participants in all intervention groups, and often shared with relevant colleagues. Some hospitals did not report ongoing monitoring of practice locally. However, some re-monitored weekly or monthly using a mix of formal and informal audits. Although enactment of target behaviours was evident, participants did not explicitly refer to using the enhanced reports or toolkit to facilitate these processes (see Report Supplementary Material 25). It is, therefore, not possible to attribute enactment to the enhanced interventions.
Context
Web analytics
The NCA website tracked downloads of all documents in the document library for each site, including non-trial-related documents such as reports from previous audits and data collection tools for other ongoing or planned audits. Figure 12 shows the number of non-trial documents downloaded by all clusters during trial 1. This large number of downloads suggests that hospitals were often engaging with materials from other transfusion audits, representing potential competing priorities that could detract from engagement with the trial audits.
FIGURE 12.
Number of downloads of non-trial documents for 8 months following intervention delivery.
Interviews
Several contextual factors were identified during the interviews with participants from all trial arms that may have undermined perceptions of, and response to, the feedback (see Report Supplementary Material 26). Many of these related to the audit side of the NCA audit and feedback intervention. The enhanced interventions in AFFINITIE focused on the feedback component and did not intervene with or attempt to enhance the audit component, which was standardised across all trial arms (i.e. same audit standards and data collection procedures).
Many participants discussed how some audit standards were unrealistic and did not represent the complexity of blood transfusion practice, particularly for different types of surgery (trial 1). Audit standards were also perceived as inflexible and not accounting for clinical judgement and knowledge when decisions to transfuse were made. In trial 2, strong concerns were expressed about the lack of evidence underpinning certain audit standards. Participants argued that adherence to such standards would be inappropriate. Consequently, several participants reported low motivation to change their transfusion practice, despite performing poorly against these standards.
These issues were further compounded by perceived issues around the credibility of the audit data, because of findings based on a small number of patient cases, the inappropriate inclusion of certain patient groups (e.g. fractured neck of femur patients in elective surgery audit) and scepticism around how performance against standards was assessed. Some viewed the audit data collection process as complicated, burdensome and confusing, sometimes leading to inaccurate data being collected and resulting in erroneous performance feedback being delivered.
Many participants reported that colleagues had varying levels of interest in blood transfusion. Unsurprisingly, transfusion practitioners and haematologists viewed transfusion as a core part of their clinical role/specialty and, thus, more of a priority. However, for clinicians from other specialties (e.g. surgery, anaesthetics) transfusion was a relatively smaller part of their role and, thus, of lower priority. This translated to mixed willingness to change practice and transfusion practitioners feeling as though they had limited influence over the practice of their colleagues (see Report Supplementary Material 26).
One theme discussed by most participants was the influence of national initiatives on transfusion practice. In 2015, NICE published new guidelines for the assessment and management of blood transfusions. These guidelines were published before the feedback for the surgical audit in trial 1 had been delivered. When participants discussed how they were responding to the feedback, and what recommendations they were working on, most participants reported how this was in response to the NICE guidlines rather than the feedback received from the audit. NICE was generally held in high esteem by clinicians and seen as more credible. Interestingly, the feedback from the NCA was seen as evidence to support the changes that they were already seeking to make based on NICE guidelines, rather than the other way around (see Report Supplementary Material 26).
Conclusions
The process evaluation demonstrated that the interventions were delivered with high fidelity, suggesting that they are feasible to deliver. Both interventions had good initial receipt, but subsequent engagement, particularly with enhanced follow-on, was low. Enactment appeared good, with hospitals across all trial arms engaging to varying extents in the target behaviours in response to feedback. Further analysis suggested that responses were driven by contextual factors, including the dissemination of national guidelines, rather than the enhanced interventions themselves.
Therefore, one interpretation is that the trials were not valid tests of the intervention, owing to low fidelity of receipt and enactment, and contextual factors that may have interfered with response to feedback. The lack of significant differences between interventions is thus to an extent unsurprising. However, although contextual factors drive practice change, as these would influence all trial arms the trial results still do not indicate that the enhanced interventions have any effect. The observed low levels of engagement with the feedback interventions also suggest that the interventions as designed were not sufficiently engaging, not feasible enough and/or potentially burdensome to use in practice. There is a need for further research to explore different ways of designing and delivering feedback to facilitate and increase engagement. Examples include automating aspects of the response to feedback (e.g. goal-setting, planning) as much as possible to reduce reliance on input from health-care professionals who have limited time and competing clinical priorities.
Nonetheless, participants reported a preference for some of the interventions, particularly enhanced content reports over standard reports, suggesting that there may still be merit in implementing and evaluating changes to the format and content of standard reports to enhance the understanding and usability of NCA feedback. This includes providing three levels of enhanced content (a brief report highlighting comparative performance and recommendations for selected key audit standards; a longer report covering all audit standards; and the long, detailed standard report).
Low engagement with feedback was in part attributed by interview participants to limitations in the upstream audit processes. This highlights the need for future research and practice to investigate enhancing the process of setting of audit standards and facilitating more accurate and efficient audit data collection.
Limitations
A methodological strength of our process evaluation was the assessment of fidelity at both the provider and the recipient level. Numerous reviews demonstrate that most fidelity assessments focus solely on delivery, and few focus on receipt and engagement. 36,37 A further strength is the combined use of in-depth qualitative and broader quantitative methods to gather process data across the range of participating clusters. This included objective measures (web analytics, content analysis of audio-recorded session transcripts) that are less prone to self-report and recall biases. Limitations include participant self-selection, reporting and recall biases in the interview data. A further limitation is that researchers involved in developing the enhanced interventions were also involved in collecting and interpreting process evaluation data and were not blind to trial arm allocation during analysis. Although this may have biased interpretation, all researchers who analysed fidelity data were blind to trial outcomes. Finally, the enhanced feedback interventions were compared against usual feedback practice by the NCABT, and, as an active control condition, this shared some elements with the enhanced interventions, which may have resulted in a loss of treatment differentiation between trial arms. 30
Workstream 4: tools for relevant audit and feedback programmes in the wider NHS
Alongside the WSs addressing the national cluster trial and the process evaluation, we planned activities to disseminate our findings. We originally proposed a series of structured stakeholder ‘roundtable’ meetings, involving, among others, clinical and management leads involved in the transfusion audits and other national audit programmes, to produce evidence-based resources to support national audit programmes to adapt and adopt the two feedback interventions developed and evaluated with our programme. As the programme progressed, we found that developing relationships and sharing feedback with a number of national audit programmes, as well as working as much as possible within existing networks, offered more fruitful approaches to engagement and dissemination than hosting further ‘roundtable’ events. We therefore changed our approach (highlighted in our 2017 progress report) to the activities outlined below, described in further detail in Appendix 5. Although we recognise that both feedback interventions (enhanced content and enhanced follow-on) were ineffective when evaluated in the trials, we have made relevant intervention materials available (see Report Supplementary Material 9–14) because they nevertheless cover important points for audit programmes to consider when designing interventions and delivering feedback.
Engagement with the Healthcare Quality Improvement Partnership and national clinical audits
We channelled the majority of our engagement activities with national audit programmes through the Healthcare Quality Improvement Partnership (HQIP), given that it is responsible for commissioning most national audits in the UK, holds regular update events and distributes guidance on audit methods. We participated in the HQIP Methodology Advisory Group from 2017 onwards. This allowed us to gain an understanding of the key methodological issues facing national audits (e.g. ensuring data validity, promoting local action following feedback). We shared emerging lessons from AFFINITIE and updates of evidence on effective feedback at HQIP seminars and with several individual audit programmes.
Audit of audits
We planned a series of audits of existing national audit programmes. We identified a baseline sample of national audit reports for 23 programmes listed on the HQIP website in November 2015. We applied a set of evidence-based and good practice criteria to these reports. We verified our assessments, where possible, with national audit leads and project managers. HQIP published Reporting for Impact Guidance, to enhance the impact of national audits in March 2016. 38 We then repeated our assessment in January 2017 by applying the criteria to a follow-up sample of 20 re-audit reports (out of the original 23 national audit programmes).
We identified a range of improvements over time in the content of audit reports, for example in the identification of key audit standards, findings and recommendations, the definition of target groups for dissemination, the use of comparators and achievable benchmarks, and the presentation and specification of action plans. We also identified areas for improvement, for example reducing time intervals between data collection and feedback. We reported our findings directly to HQIP and shared them at international collaborator meetings. With further refinements, we consider that a criterion-based ‘audit of audits’ offers one efficient means of monitoring the quality of national audit reports.
The Audit and Feedback MetaLab
We are co-founders of this international collaboration, led by our co-investigator, Jeremy Grimshaw. The Audit and Feedback MetaLab (www.ohri.ca/auditfeedback/) is an international research and health-care community that aims to synthesise and share evidence on A&F, engage with health system partners and provide a trusted source of evidence and recommendations, and develop research capacity and practical expertise in A&F. We have held annual meetings to bring together researchers and audit leaders in Europe and North America since 2014. The 2017 Leeds meeting included presentations from HQIP and national audit programmes, evidence updates and discussions about challenges faced by national audits.
Joint seminar with the Healthcare Quality Improvement Partnership: what can national clinical audits learn about improving impact from the AFFINITIE research programme?
We asked HQIP to host the main dissemination seminar for AFFINITIE because we recognised that this would lend the programme credibility and extend our reach to national audits. The seminar aimed to identify lessons for national clinical audit programmes based on AFFINITIE findings and experience.
We invited national audit leads, members of the public and researchers. Ninety-nine delegates registered for the seminar, which took place in London in June 2019. We presented the trial and process evaluation findings, contextualised our work in the wider evidence base, and sought feedback on the materials and toolkits produced as part of AFFINITIE.
Participant suggestions for national audit programmes responses largely echoed findings from the intervention development work and the process evaluation (e.g. ensuring credibility of audit measures, delivering timely feedback, offering proactive support for local teams to act on feedback findings).
Patient and public involvement
Our patient and public involvement (PPI) panel contributed to the design of the research questions, shaped our application for funding, and influenced the design of the enhanced feedback interventions. As in other implementation research, there was limited opportunity for the panel to assess the appropriateness and feasibility of the interventions targeting health-care professionals. Panel members informed implementation recommendations for the wider clinical, policy and academic community.
The panel was chaired by Alan White (co-investigator), who represented the panel in Programme Management Group meetings and at a consensus meeting to design the enhanced interventions. Alan brought valuable organisational and policy experience as a member and past chairperson of the Royal College of Physicians Patient and Carer Liaison Group, past chairperson of an NHS trust, and a patient representative involved in the National Comparative Audit of Transfusion (conducted by the Intercollegiate Committee on Haematology).
Fellow members included Phil Willan (deputy chairperson of the PPI panel), member of the Royal College of Physicians Patient and Carer Involvement Steering Group and member of an expert advisory group for the Medicines and Healthcare products Regulatory Agency, who brought experience of having a transplant and blood transfusions; Graham Prestwich, a lay member of NHS Leeds North Clinical Commissioning Group Patient and Public Involvement Group; Pauline Bland, a community development worker in Bradford; and Ella Reeves, a Patient Experience and Involvement Manager at John Radcliffe Hospital. Liz Glidewell (co-investigator) supported the panel to engage with the WS leads.
Derek Calum contributed as an independent PPI representative member of the Trial Steering Group.
Significant contributions were made as identified in a framework for the role of patients and the public in implementation research39 (Box 1).
-
Guided a change in the primary outcome measure.
-
Advised on methods to recruit hospital trusts. Panel members supported streamlined regional (rather than trust) processes for local governance review and provided valuable contextual information on the research setting.
-
Reviewed and commented on the funding application as a co-investigator.
-
Evaluated prototype intervention content and promoted the following changes: foregrounding the patient in feedback messages; considering if feedback would be badged and by whom; prioritised the need for rapid feedback to health-care professionals; suggested moving the sign-off box to the front page to increase accountability; suggested developing a ward poster as part of the enhanced follow-on intervention; and emphasised the need for coaching alongside the enhanced delivery intervention that informed the decision to use telephone support to maximise participant engagement.
-
Commented on training materials developed to support the intervention.
-
Attended a multidisciplinary consensus panel meeting to represent the views of patients in designing the enhanced interventions.
-
Pre-tested the content of research materials. Alan White supported the development of a questionnaire for data collection. The panel advised on clear labelling of materials to research participants.
-
Set the agenda for PPI meetings in collaboration with WS leads.
-
Reviewed interim analyses and trial findings, raised the importance of displaying information in an easy-to-understand format.
-
Co-authored the Plain English summary.
-
Provided unique knowledge through experience of working closely with targeted transfusion teams.
-
Provided personal insight into how feedback interventions may be received by professionals and hospital management.
-
Guided the direction of future research to improve audit more widely in the NHS. Dissemination activities involved end-users including those commissioning and delivering other national NHS audits, particularly the National Clinical Audit and Patient Outcomes Programme clinical leads. The research team engaged more widely with international audit leaders, policy-makers and academics at annual A&F collaborative meetings (later the Audit and Feedback MetaLab) in Canada, the UK and the Netherlands.
The panel welcomed the research and commented on their support for conducting research within further national audit programmes. Panel members continually expressed a willingness to contribute but reported that there were limited opportunities for PPI input throughout the programme of work given that audit standards were prespecified and patients were not the target of the intervention.
Conclusions
We have undertaken a robust evaluation of a national A&F programme in transfusion through the successful conduct of two linked national cluster-randomised trials. We identified that current blood transfusion A&F in England makes limited use of available theory and evidence about how to effectively design and deliver A&F. We designed and implemented two behaviourally modified interventions aimed at augmenting feedback by enhancing the content of the reports and follow-on support at hospitals. Modelling indicated that the enhanced feedback interventions could be inexpensive per hospital and acceptable to recipients, but they were found to be no more effective than standard feedback in increasing the acceptable use of blood.
The process evaluation explored reasons for the lack of impact of the interventions on the acceptable use of blood, and identified a lack of credibility of the audit standards and data validity, and low engagement with the audit and feedback cycle. Levels of fidelity to the enactment of feedback were variable after the initial phase, and often poor at hospital sites, with a lack of sustained interest in telephone support or accessing the follow-on materials. Other contextual issues included variable interest in transfusion that was highlighted by health-care professionals, including how to use the feedback to change the behaviour of remote transfusion prescribing clinicians. Overall, it seems likely that the lack of any effect of the enhancements was driven, in part, by factors outside the reach of the interventions.
National clinical audit programmes have a significant potential to change practice. However, the potential for feedback to deliver change depends on the integrity and validity of the upstream steps of setting standards and data quality. There is also a need to devote greater attention to understanding engagement and implementation at local levels. It may well be that our low-cost interventions have merit to enhance feedback in other contexts, but our robust assessment as successfully delivered was unable to identify any effect within a national audit of blood transfusion.
Limitations
Several factors limited the interpretation of the cluster randomised trials, including the number of participating clusters, the number of patient records audited per cluster, the effect of randomised clusters lost to follow-up, the use of complex audit data, and use of audit standards requiring the greatest change in practice applying to only small numbers of audited patients.
The assessment of fidelity at both the provider and the recipient level was a methodological strength of our process evaluation. Numerous reviews show that most fidelity assessments focus solely on delivery and not on receipt and engagement. The combined use of in-depth qualitative and broader quantitative methods to gather process data across the range of participating clusters was a further strength. We included objective measures (web analytics, content analysis of audio-recorded session transcripts) that are less prone to self-report and recall biases. Limitations included participant self-selection, reporting and recall biases in the interview data.
Reflections on the programme
AFFINITIE is an example of an ‘implementation laboratory’ that involves close collaboration between a health system delivering an implementation strategy at scale and a research team. 31 Working with an existing national audit programme allowed us to efficiently conduct head-to-head trials comparing different feedback interventions that are needed but relatively sparse in the A&F literature. 5 We invested considerable effort in integrating research within the NCABT and also appreciated its reciprocity in changing arrangements for the development of feedback reports and adjusting timing of data collection and feedback to align with the randomised trials. We would advise others not to underestimate the levels of communications and goodwill required to embed research within a national audit programme. AFFINITIE allowed a close examination of the strengths and limitations of the NCABT that may be relevant to other national audit programmes and the major challenges involved in a rolling programme that selects one or two new topics per year for national audits. This contrasts with several other national audit programmes that consistently focus on a core, limited set of indicators. The NCABT can address a wide variety of priorities, but perhaps at a cost of continually focusing on a more limited range of key, evidence-based audit criteria. This may underpin some of the issues with credibility of data and explain some of the limited progress made in recent years in reducing the number of unnecessary blood transfusions. We also noted that the algorithms used to assess appropriateness of transfusions were sometimes complex. Clarity of audit standards is fundamental to all stages of effective audit delivery, and in turn these have an impact on the design of algorithms applied to assess the appropriate use of interventions. It was not always clear how these algorithms were validated and, hence, whether or not they would consistently assign all transfusions as appropriate.
AFFINITIE also acted as a focus for discussions about how to improve impact with HQIP and other national audit programmes. The wide coverage of and high levels of hospital participation in the NCABT underpinned ‘real-world’ generalisability and the programme funding allowed a parallel mixed-methods process evaluation to provide critical and unique insights into the trials findings, which can inform further research and practice.
Implications for health care
We emphasise that there is still a firm evidence base underpinning A&F, including different approaches to enhance its effects on patient care on which national audits can draw. Our work has demonstrated ways of making feedback reports more accessible to recipients, but they appear unlikely to work in the absence of more favourable contexts, for example where audit data are perceived as more valid and reliable indicators of performance. Components of our interventions, such as providing three levels of enhanced content (a brief report highlighting comparative performance and recommendations for selected key audit standards; a longer report covering all audit standards; and the long, detailed standard report), might have broader applicability for national audit leaders. Our process evaluation highlights the need for national audit teams to define and enhance the key processes of setting clear audit standards, strengthen the accuracy and efficiency of audit data collection, and develop feedback interventions that can better support meaningful recipient engagement and enactment.
Future practice aiming to improve the effect of the NCABT could consider ways of strengthening the perceived credibility and relevance of feedback (e.g. by reducing the interval between data collection and delivery of feedback) and enabling more effective local responses.
We note from our economic modelling that the intervention costs per site were modest; this means that the intervention would need to have only a small impact on the volume of blood transfused for it to be cost neutral. However, it should also be borne in mind that, as shown in the trials, there was no evidence of a clinically or statistically significant effect on acceptable transfusions. Initiatives such as the international Audit and Feedback MetaLab provide a forum for the commissioners and providers of national clinical audits to interact with the research community and keep up to date with emerging research evidence.
Recommendations for research
-
We have demonstrated the feasibility of embedding ambitious large-scale and rigorous research within national clinical audit programmes. Further head-to-head comparisons of different feedback interventions are needed within these programmes to identify cost-effective ways of increasing the impact that the interventions have on changing practice.
-
Future studies could develop and evaluate interventions to promote meaningful recipient engagement and support focused local action in response to feedback.
-
Pilot studies to ensure sufficient fidelity and identify likely effective ‘doses’ of feedback interventions may increase the likelihood of definitive trials being able to investigate cost-effectiveness robustly.
-
Future health economics work could include a patient-level analysis of costs and QALYs to move beyond a process outcome such as percentage of acceptable transfusions (which does not translate as readily into meaningful economic benefit.) A future value-of-information analysis may be used to identify key uncertainties around cost parameters and adverse events associated with acceptable compared with not acceptable transfusions.
Acknowledgements
We thank all of the hospitals and transfusion departments and teams who participated in this programme and our contributors to the PPI panel. We acknowledge the specific work of the audit leads across the two audit cycles (Dr K Pendry, Dr S Allard, Dr J Burchill and Dr M Karakantza), and thank Professor M Murphy for constructive input into early stages of the programme development. Isabelle Smith, Thomas Morris, Zoe Craig and Ellen Mason contributed to the analysis in WS2 and Karla Diaz-Ordaz contributed to the statistical analysis plan for WS2. We would also like to acknowledge Tasneem Khan, NIHR Academic Clinical Fellow in General Practice, who co-designed, conducted and co-analysed the ‘audit of audits’ survey.
Contributions of authors
Robbie Foy (https://orcid.org/0000-0003-0605-7713) co-led workstream 2 and led workstream 4.
Fabiana Lorencatto (https://orcid.org/0000-0003-4418-7957) and Natalie Gould conducted the interview work and process evaluation.
Rebecca Walwyn (https://orcid.org/0000-0001-9120-1438) was the statistical lead.
Jill Francis (https://orcid.org/0000-0001-5784-8895) led workstreams 1 and 3.
Stephen McIntyre (https://orcid.org/0000-0001-9394-8914), Riya Patel (https://orcid.org/0000-0001-6572-5924), James Smith (https://orcid.org/0000-0002-0448-8774), Camilla During and Jon Bird (https://orcid.org/0000-0002-1681-1532) contributed to programme and trial data collection and analysis.
Suzanne Hartley (https://orcid.org/0000-0003-2346-9461) was responsible for operational delivery of the trials.
Robert Cicero (https://orcid.org/0000-0002-3713-2934) contributed to the randomisation and analysis, with guidance from Rebecca Walwyn and Amanda Farrin (https://orcid.org/0000-0002-2876-0584).
Liz Glidewell (https://orcid.org/0000-0003-2519-2654) provided support for analysis of contamination effects and support for PPI.
John Grant-Casey (https://orcid.org/0000-0001-5166-4550) managed the National Comparative Audit programme in transfusion alongside the conduct of the trials, with support from Megan Rowley (https://orcid.org/0000-0002-9322-8527) and Simon J Stanworth.
Alison Deary (https://orcid.org/0000-0001-8351-4186) advised on governance and data acquisition and programme financial support.
Nicholas Swart (https://orcid.org/0000-0001-8343-766X) with guidance from Stephen Morris (https://orcid.org/0000-0002-5828-3563) conducted the economic evaluation.
Michelle Collinson (https://orcid.org/0000-0003-3568-6455) led the statistical programming for the enhanced content intervention.
Lauren Moreau (https://orcid.org/0000-0002-0280-6345) provided invaluable administration support.
Susan Michie (https://orcid.org/0000-0003-0063-6378) and Jeremy M Grimshaw (https://orcid.org/0000-0001-8015-8243) provided overall programme direction and advice at multiple steps.
Simon J Stanworth was principal investigator for the programme and co-led workstream 2.
Publications
Gould NJ, Lorencatto F, Stanworth SJ, Michie S, Prior ME, Glidewell L, et al. Application of theory to enhance audit and feedback interventions to increase the uptake of evidence-based transfusion practice: an intervention development protocol. Implement Sci 2014;9:92. https://doi.org/10.1186/s13012-014-0092-1
Lorencatto F, Gould NJ, McIntyre SA, During C, Bird J, Walwyn R, et al. A multidimensional approach to assessing intervention fidelity in a process evaluation of audit and feedback interventions to reduce unnecessary blood transfusions: a study protocol. Implement Sci 2016;11:163. https://doi.org/10.1186/s13012-016-0528-x
Hartley S, Foy R, Walwyn REA, Cicero R, Farrin AJ, Francis JJ, et al. The evaluation of enhanced feedback interventions to reduce unnecessary blood transfusions (AFFINITIE): protocol for two linked cluster randomised factorial controlled trials. Implement Sci 2017;12:84. https://doi.org/10.1186/s13012-017-0614-8
Gould NJ, Lorencatto F, During C, Rowley M, Glidewell L, Walwyn R, et al. How do hospitals respond to feedback about blood transfusion practice? A multiple case study investigation. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0206676
Grimshaw JM, Ivers N, Linklater S, Foy R, Francis JJ, Gude WT, et al. Reinvigorating stagnant science: implementation laboratories and a meta-laboratory to efficiently advance the science of audit and feedback. BMJ Qual Saf 2019;28:416–23. https://doi.org/10.1136/bmjqs-2018-008355
Stanworth SJ, Lorencatto F, Gould N, Grant-Casey J, Deary A, Hartley S, et al. Can we do better? Bridging the research to practice gap in patient blood management–optimizing ‘audit & feedback’ and the challenges of undertaking a national cluster-randomized controlled trial. VOXS 2019;14:129–35.
Stanworth SJ, Walwyn R, Grant-Casey J, Hartley S, Moreau L, Lorencatto F, et al. ; AFFINITIE Collaborators. Effectiveness of enhanced performance feedback on appropriate use of blood transfusions: a comparison of 2 cluster randomized trials. JAMA Netw Open 2022;5:e220364. https://doi.org/10.1001/jamanetworkopen.2022.0364
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Stanworth SJ, Walwyn R, Grant-Casey J, Hartley S, Moreau L, Lorencatto F, et al. AFFINITIE Collaborators . Effectiveness of enhanced performance feedback on appropriate use of blood transfusions: a comparison of 2 cluster randomized trials. JAMA Netw Open 2022;5. https://doi.org/10.1001/jamanetworkopen.2022.0364.
- Hartley S, Foy R, Walwyn REA, Cicero R, Farrin AJ, Francis JJ, et al. The evaluation of enhanced feedback interventions to reduce unnecessary blood transfusions (AFFINITIE): protocol for two linked cluster randomised factorial controlled trials. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0614-8.
- Cane J, Richardson M, Johnston M, Ladha R, Michie S. From lists of behaviour change techniques (BCTs) to structured hierarchies: comparison of two methods of developing a hierarchy of BCTs. Br J Health Psychol 2015;20:130-50. https://doi.org/10.1111/bjhp.12102.
- Murphy MF, Waters JH, Wood EM, Yazer MH. Transfusing blood safely and appropriately. BMJ 2013;347. https://doi.org/10.1136/bmj.f4303.
- Ivers NM, Sales A, Colquhoun H, Michie S, Foy R, Francis JJ, et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-14.
- Carver CS, Scheier MF. On the Self-regulation of Behavior. Cambridge: Cambridge University Press; 2001.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Gardner B, Whittington C, McAteer J, Eccles MP, Michie S. Using theory to synthesise evidence from behaviour change interventions: the example of audit and feedback. Soc Sci Med 2010;70:1618-25. https://doi.org/10.1016/j.socscimed.2010.01.039.
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6. https://doi.org/10.1002/14651858.CD000259.pub3.
- Presseau J, McCleary N, Lorencatto F, Patey AM, Grimshaw JM, Francis JJ. Action, actor, context, target, time (AACTT): a framework for specifying behaviour. Implement Sci 2019;14. https://doi.org/10.1186/s13012-019-0951-x.
- Steinmo SH, Michie S, Fuller C, Stanley S, Stapleton C, Stone SP. Bridging the gap between pragmatic intervention design and theory: using behavioural science tools to modify an existing quality improvement programme to implement ‘Sepsis Six’. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0376-8.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Gould NJ, Lorencatto F, Stanworth SJ, Michie S, Prior ME, Glidewell L, et al. Application of theory to enhance audit and feedback interventions to increase the uptake of evidence-based transfusion practice: an intervention development protocol. Implement Sci 2014;9. https://doi.org/10.1186/s13012-014-0092-1.
- Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363-74. https://doi.org/10.2307/2529786.
- Gould NJ, Lorencatto F, During C, Rowley M, Glidewell L, Walwyn R, et al. How do hospitals respond to feedback about blood transfusion practice? A multiple case study investigation. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0206676.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0605-9.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psych 2008;57:660-80. https://doi.org/10.1111/j.1464-0597.2008.00341.x.
- van Someren MW, Barnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- NHS Blood and Transplant . NHSBT Pricing Proposals for 2017–18 2016. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/2188/16-80.pdf (accessed 9 September 2021).
- National Institute for Health and Care Excellence (NICE) . Costing Statement: Blood Transfusion. Implementing the NICE Guideline on Blood Transfusion [NG24] 2015.
- Royal College of Nursing . NHS Pay Scales 2017–2018 2018. https://rcn.org.uk/employment-and-pay/nhs-pay-scales-2017-18 (accessed 1 January 2018).
- British Medical Association . Pay Scales for Junior Doctors 2017 2018 2018. https://bma.org.uk/advice/employment/pay/juniors-pay-england (accessed 1 January 2018).
- British Medical Association . Pay Scales for Consultants 2017 2018 2018. https://bma.org.uk/advice/employment/pay/juniors-pay-england (accessed 1 January 2018).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:S52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Grimshaw JM, Ivers N, Linklater S, Foy R, Francis JJ, Gude WT, et al. Reinvigorating stagnant science: implementation laboratories and a meta-laboratory to efficiently advance the science of audit and feedback. BMJ Qual Saf 2019;28:416-23. https://doi.org/10.1136/bmjqs-2018-008355.
- Lorencatto F, Gould NJ, McIntyre SA, During C, Bird J, Walwyn R, et al. A multidimensional approach to assessing intervention fidelity in a process evaluation of audit and feedback interventions to reduce unnecessary blood transfusions: a study protocol. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0528-x.
- Witteman HO, Presseau J, Nicholas Angl E, Jokhio I, Schwalm JD, Grimshaw JM, et al. Negotiating tensions between theory and design in the development of mailings for people recovering from acute coronary syndrome. JMIR Hum Factors 2017;4. https://doi.org/10.2196/humanfactors.6502.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Walton H, Spector A, Tombor I, Michie S. Measures of fidelity of delivery of, and engagement with, complex, face-to-face health behaviour change interventions: a systematic review of measure quality. Br J Health Psychol 2017;22:872-903. https://doi.org/10.1111/bjhp.12260.
- Rixon L, Baron J, McGale N, Lorencatto F, Francis J, Davies A. Methods used to address fidelity of receipt in health intervention research: a citation analysis and systematic review. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1904-6.
- Healthcare Quality Improvement Partnership (HQIP) . National Clinical Audit and Patient Outcomes Programme: Reporting for Impact Guidance 2016.
- Gray-Burrows KA, Willis TA, Foy R, Rathfelder M, Bland P, Chin A, et al. Role of patient and public involvement in implementation research: a consensus study. BMJ Qual Saf 2018;27:858-64. https://doi.org/10.1136/bmjqs-2017-006954.
Appendix 1 Clinical algorithms for the primary outcome
Trial 1: pre-/postoperative transfusions
Acceptable
A pre- and/or postoperative transfusion carried out if the patient had active bleeding or the patient’s haemoglobin level fell below acceptable levels (i.e. 70 g/l if the patient did not have acute coronary ischaemia or 80 g/l if the patient had acute coronary ischaemia) using an up-to-date haemoglobin result (i.e. no older than 72 hours preoperatively or 12 hours postoperatively).
Outside guidelines
A preoperative and/or postoperative transfusion not carried out as above.
Trial 2: red blood cell/platelet transfusions
FIGURE 13.
Algorithm for RBC/platelet transfusions.
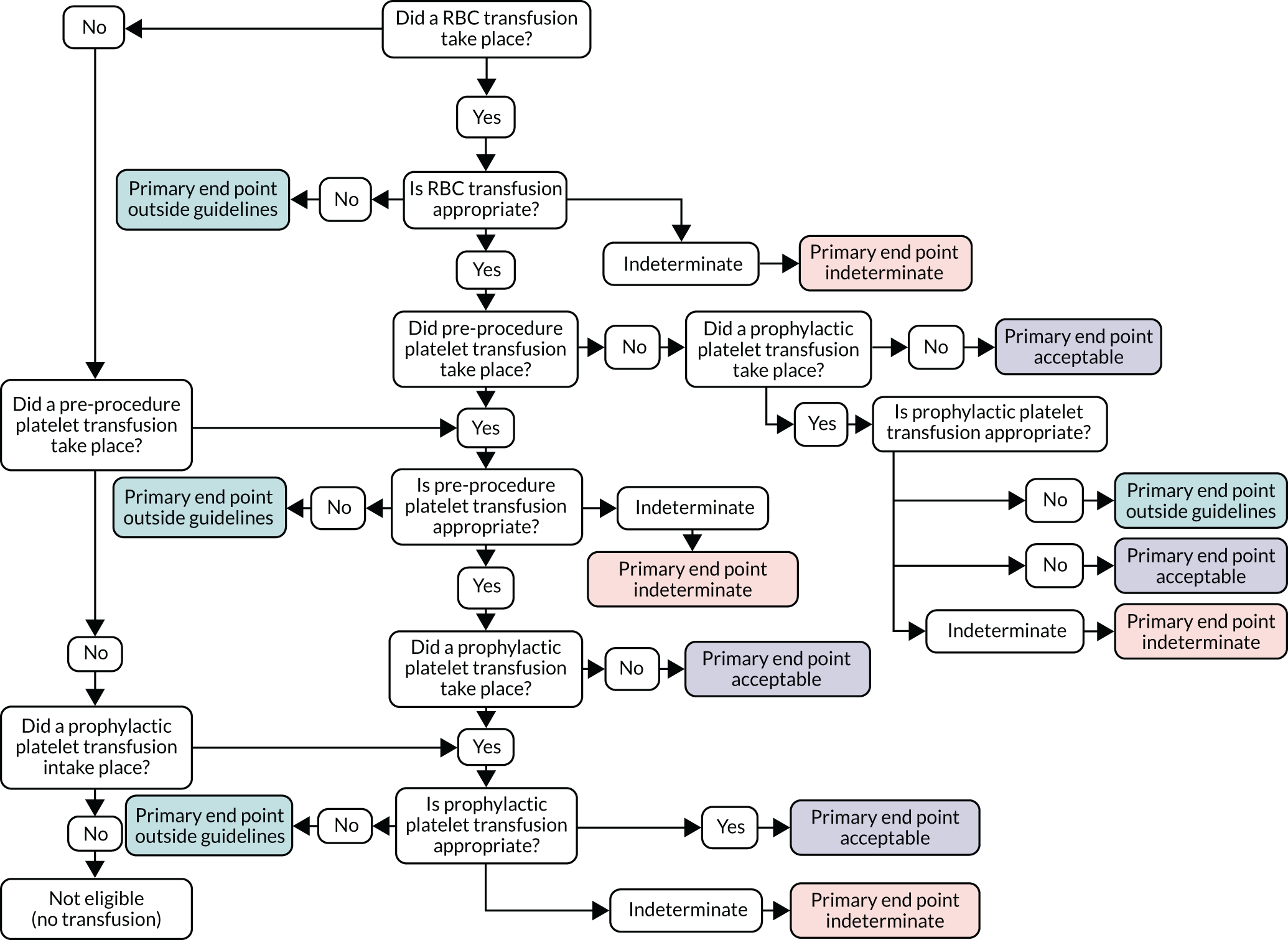
FIGURE 14.
Algorithm for RBC transfusions. Tx, transfusion.
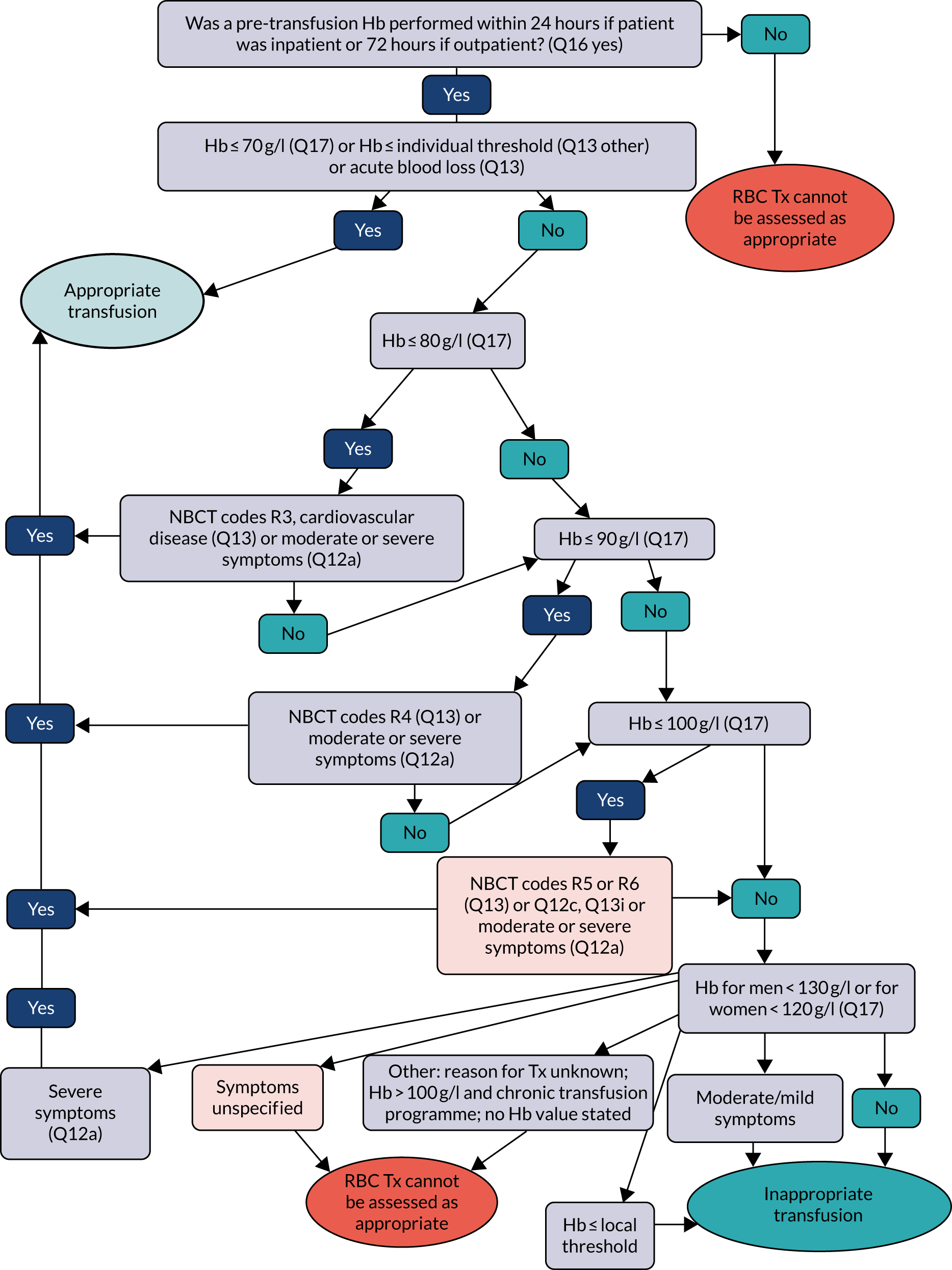
FIGURE 15.
Pre-procedure platelet transfusion current algorithm (response to Q24b). a, Essential for a platelet transfusion threshold/safe platelet count to be documented in the notes if it differs from the general guidelines. This allows adequate communication between haematologists, surgeons, anaesthetists and radiologists. Q45 gives reason why transfusion threshold was altered. b, No threshold guidance in BCSH guidelines; threshold of 50 × 109/l for endoscopy plus biopsy. Tx, transfusion.
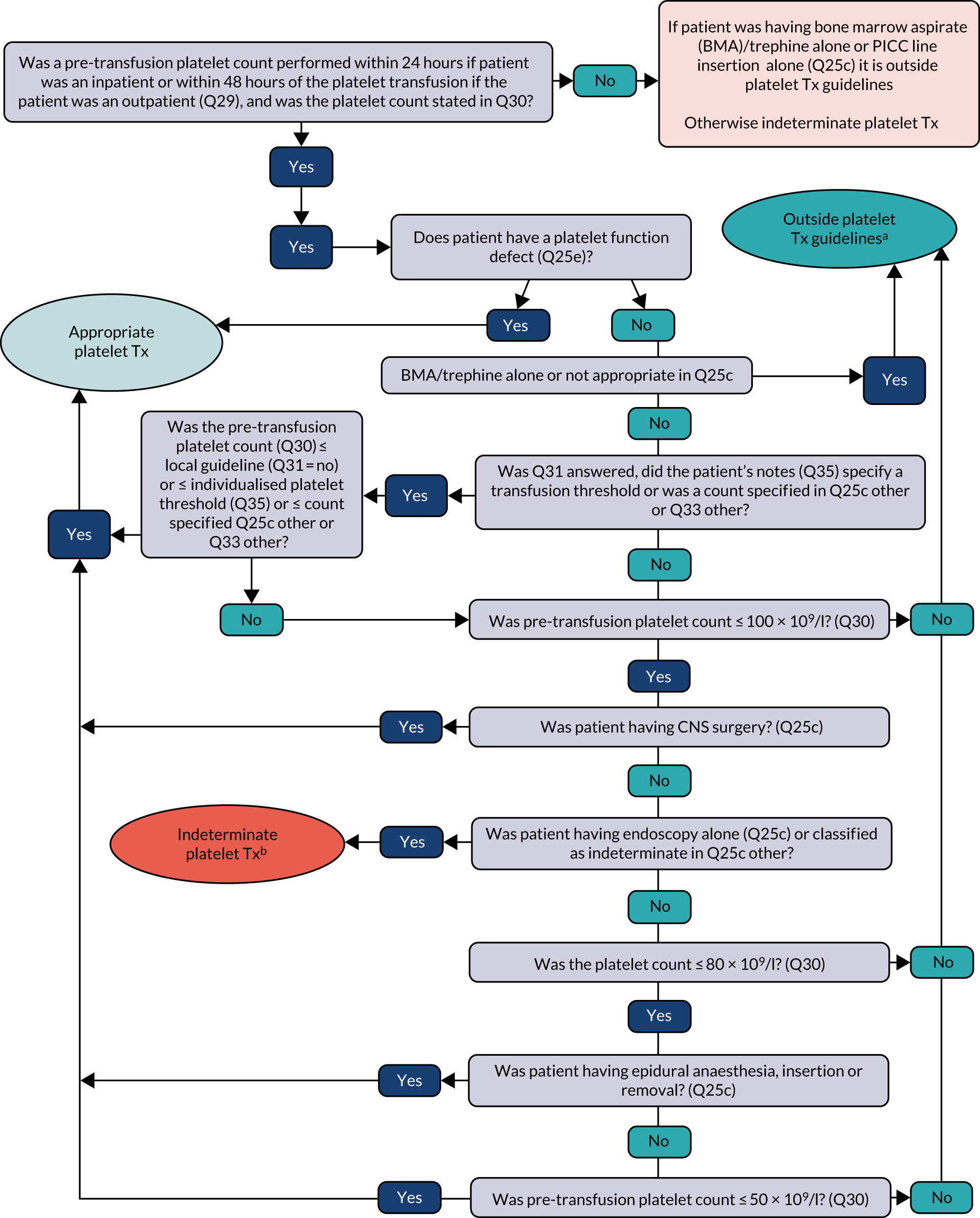
FIGURE 16.
Prophylactic platelet transfusion algorithm reversible BMF (response to Q24a). Tx, transfusion.
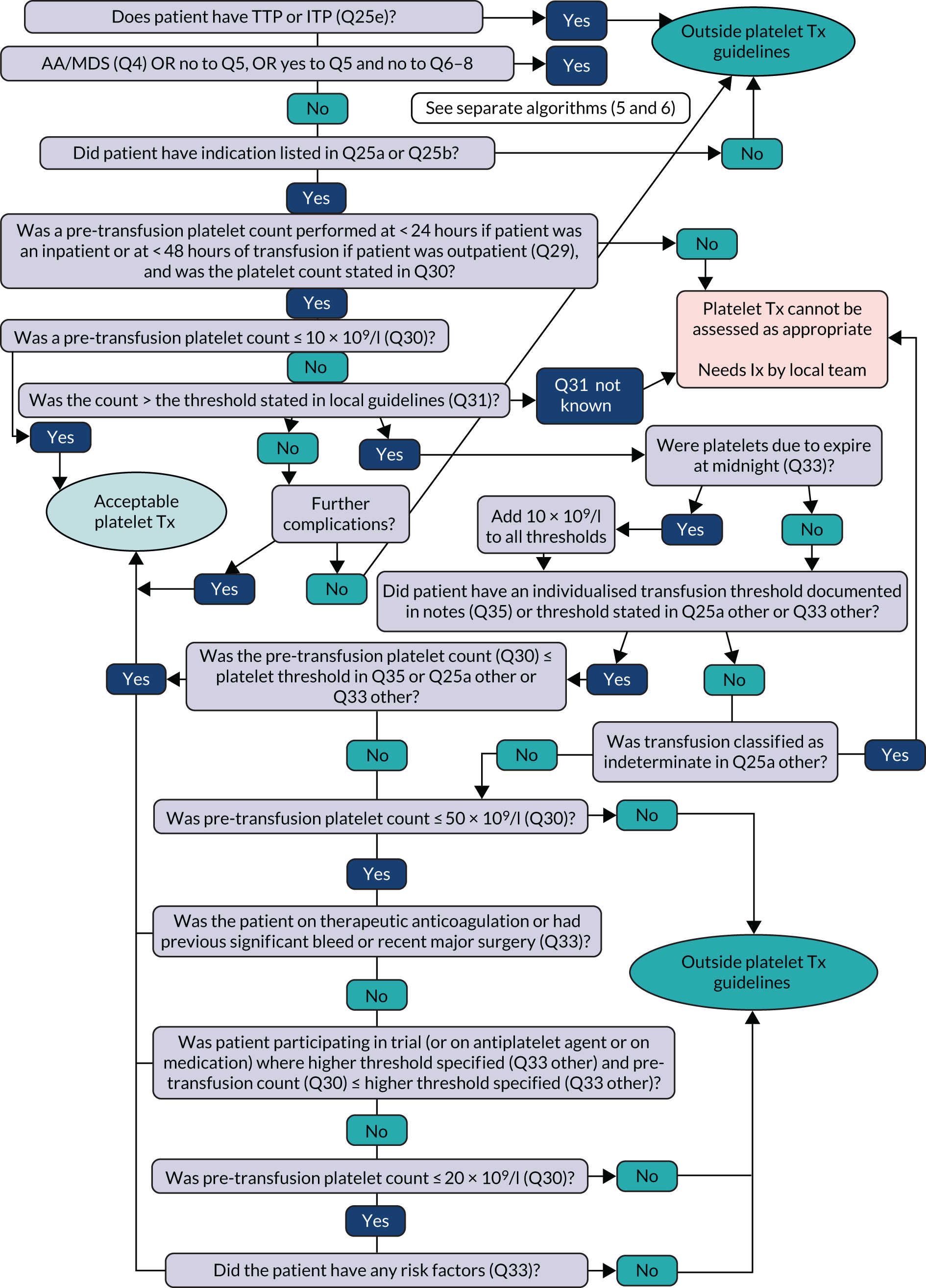
FIGURE 17.
Prophylaxis for aplastic anaemia/MDS/transfusion support only: non-intensive (N = 639). Tx, transfusion.
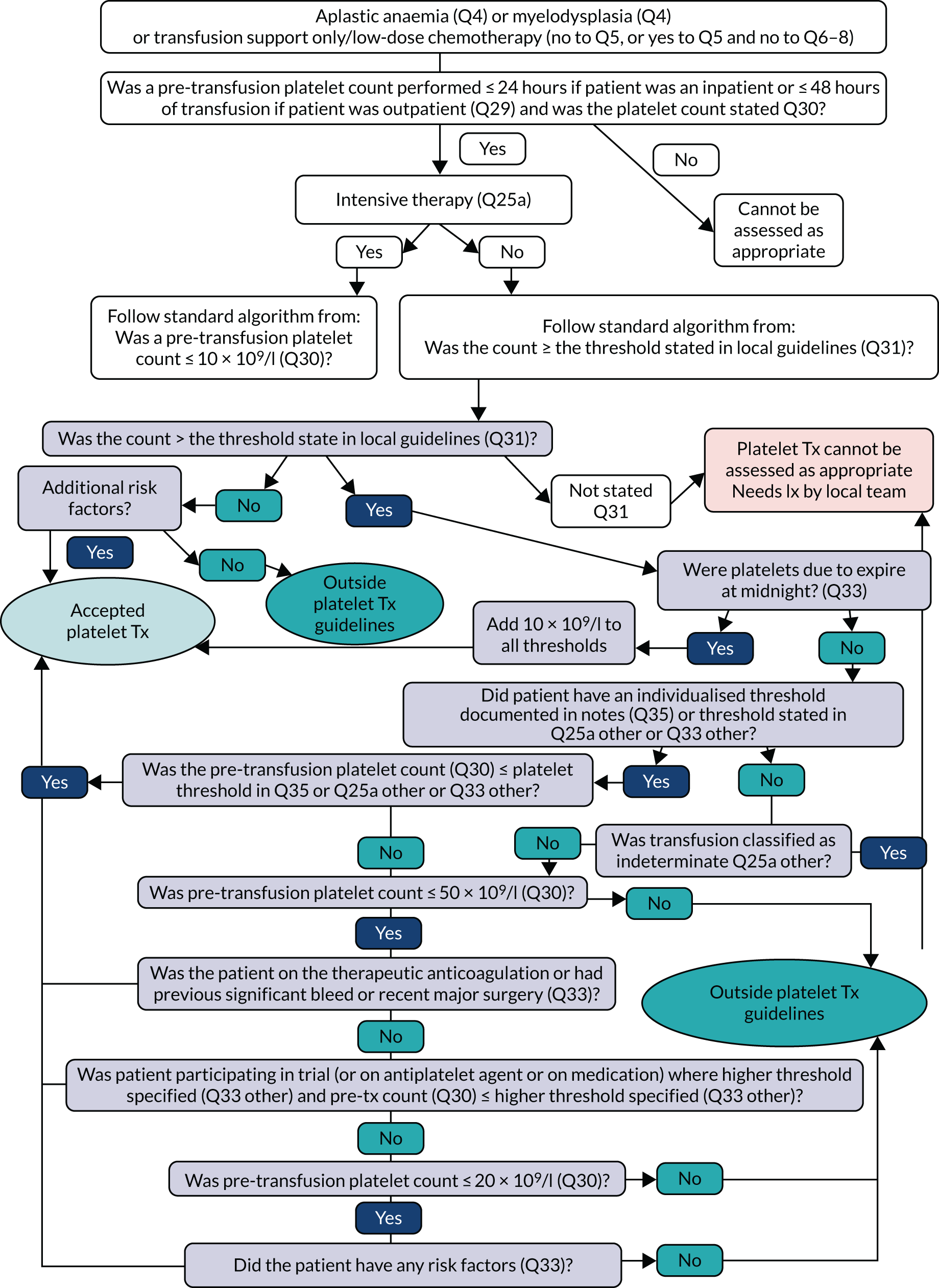
FIGURE 18.
Prophylaxis for aplastic anaemia/MDS/transfusion support only: intensive (N = 31). Tx, transfusion.
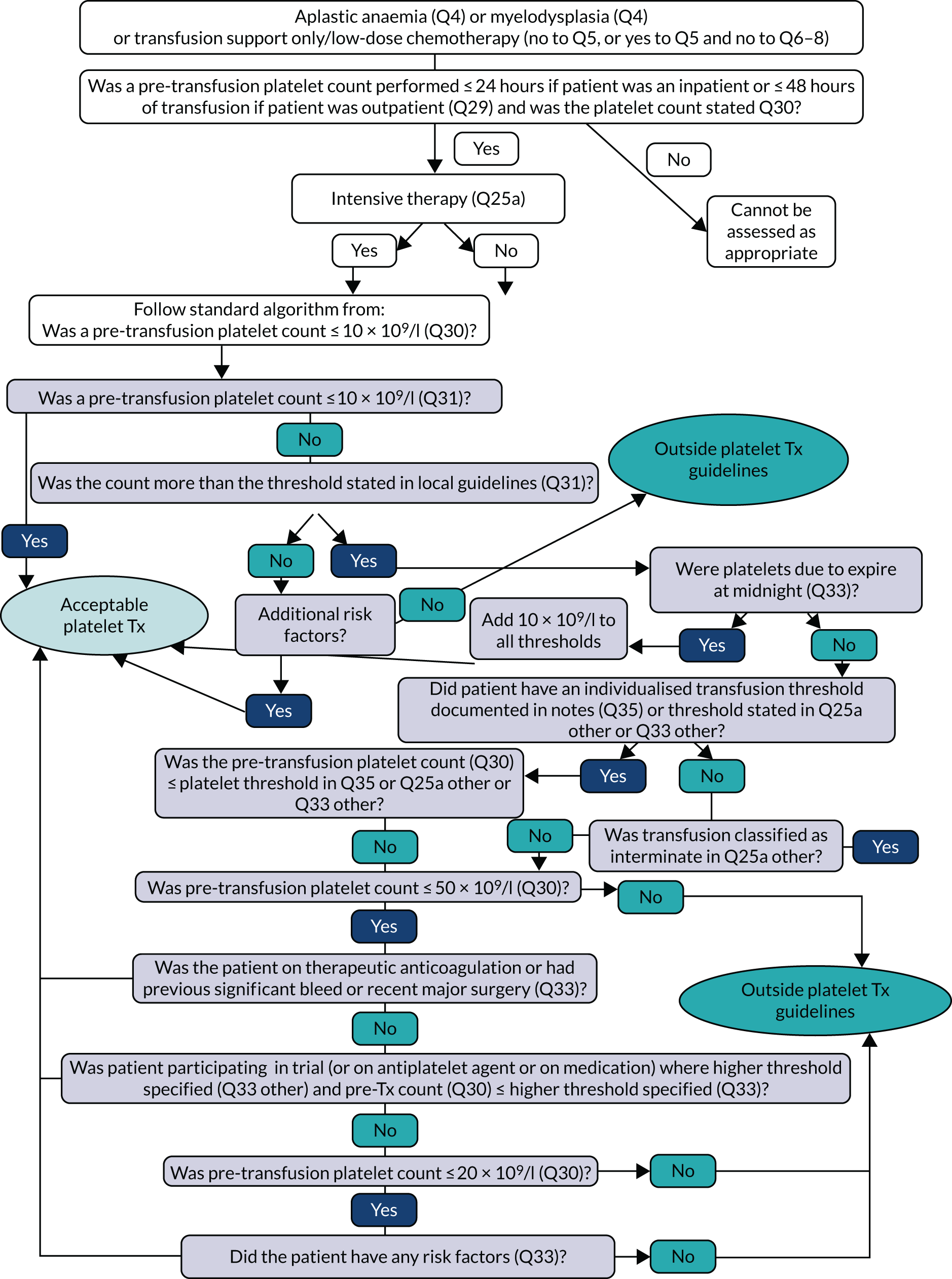
Appendix 2 Trial analysis
| Variable | Content | Follow-on | Total (N = 2714) | ||
|---|---|---|---|---|---|
| Standard (N = 1322) | Enhanced (N = 1392) | Standard (N = 1306) | Enhanced (N = 1408) | ||
| Age (years), mean (SD), n | 74.7 (13.80), 1318 | 75.1 (14.13), 1383 | 75.3 (13.80), 1302 | 74.6 (14.12), 1399 | 74.9 (13.97), 2701 |
| Gender (male), n (%) | 435 (32.9) | 470 (33.8) | 418 (32.0) | 487 (34.6) | 905 (33.3) |
| Surgical procedure, n (%) | |||||
| Orthopaedic | 444 (33.6) | 484 (34.8) | 435 (33.3) | 493 (35.0) | 928 (34.2) |
| Cardiac | 233 (17.6) | 222 (15.9) | 222 (17.0) | 233 (16.5) | 455 (16.8) |
| Fractured neck of femur | 421 (31.8) | 418 (30.0) | 410 (31.4) | 429 (30.5) | 839 (30.9) |
| Other | 222 (16.8) | 258 (18.5) | 234 (17.9) | 246 (17.5) | 480 (17.7) |
| Missing | 2 (0.2) | 10 (0.7) | 5 (0.4) | 7 (0.5) | 12 (0.4) |
| Attendance at preoperative clinic, n (%) | |||||
| Yes | 839 (63.5) | 922 (66.2) | 841 (64.4) | 920 (65.3) | 1761 (64.9) |
| No: orthopaedic | 40 (3.0) | 51 (3.7) | 44 (3.4) | 47 (3.3) | 91 (3.4) |
| No: cardiac | 46 (3.5) | 49 (3.5) | 38 (2.9) | 57 (4.0) | 95 (3.5) |
| No: fractured neck of femur | 371 (28.1) | 301 (21.6) | 357 (27.3) | 315 (22.4) | 672 (24.8) |
| No: other type of surgery | 24 (1.8) | 33 (2.4) | 25 (1.9) | 32 (2.3) | 57 (2.1) |
| No: surgery type missing | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Missing | 2 (0.2) | 35 (2.5) | 1 (0.1) | 36 (2.6) | 37 (1.4) |
| Haemoglobin level at clinic (g/l), mean (SD), n | 122.9 (17.54), 477 | 121.9 (16.57), 426 | 122.3 (16.60), 415 | 122.5 (17.51), 488 | 122.4 (17.09), 903 |
| Haemoglobin level prior to surgery (g/l), mean (SD), n | 117.4 (17.84), 1289 | 116.3 (17.18), 1305 | 117.6 (17.11), 1260 | 116.1 (17.87), 1334 | 116.8 (17.52), 2594 |
| Haemoglobin level on day 1 post surgery (g/l), mean (SD), n | 89.2 (12.59), 1137 | 90.7 (13.52), 1151 | 90.6 (12.81), 1105 | 89.3 (13.30), 1183 | 90.0 (13.08), 2288 |
| Prescribed tranexamic acid, n (%) | 454 (34.3) | 440 (31.6) | 425 (32.5) | 469 (33.3) | 894 (32.9) |
| Surgery complications, n (%) | 328 (24.8) | 381 (27.4) | 326 (25.0) | 383 (27.2) | 709 (26.1) |
| Patient died, n (%) | 49 (3.7) | 63 (4.5) | 61 (4.7) | 51 (3.6) | 112 (4.1) |
| Preoperative transfusion conducted, n (%) | 120 (9.1) | 129 (9.3) | 114 (8.7) | 135 (9.6) | 249 (9.2) |
| Intraoperative transfusion conducted, n (%) | 179 (13.5) | 184 (13.2) | 171 (13.1) | 192 (13.6) | 363 (13.4) |
| Postoperative transfusion conducted, n (%) | 1245 (94.2) | 1315 (94.5) | 1235 (94.6) | 1325 (94.1) | 2560 (94.3) |
| Preoperative blood transfusions | |||||
| N | 120 | 129 | 114 | 135) | 249 |
| Haemoglobin level (g/l), mean (SD), n | 82.8 (10.62), 115 | 84.0 (12.75), 123 | 84.1 (12.42), 110 | 82.8 (11.18), 128 | 83.4 (11.76), 238 |
| Professional making the decision to transfuse, n (%) | |||||
| Nurse | 1 (0.8) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (0.4) |
| Consultant | 55 (45.8) | 50 (38.8) | 47 (41.2) | 58 (43.0) | 105 (42.2) |
| Other doctor | 51 (42.5) | 64 (49.6) | 50 (43.9) | 65 (48.1) | 115 (46.2) |
| Other | 7 (5.8) | 1 (0.8) | 5 (4.4) | 3 (2.2) | 8 (3.2) |
| Missing | 6 (5.0) | 14 (10.9) | 11 (9.6) | 9 (6.7) | 20 (8.0) |
| Number of units transfused, n (%) | |||||
| One | 26 (21.7) | 26 (20.2) | 19 (16.7) | 33 (24.4) | 52 (20.9) |
| Two or more | 93 (77.5) | 101 (78.3) | 94 (82.5) | 100 (74.1) | 194 (77.9) |
| Missing | 1 (0.8) | 2 (1.6) | 1 (0.9) | 2 (1.5) | 3 (1.2) |
| Number of units transfused, mean (SD), n | 2.0 (0.74), 119 | 2.2 (1.04), 127 | 2.1 (0.80), 113 | 2.0 (1.00), 133 | 2.1 (0.91), 246 |
| Record of acute coronary ischaemia, n (%) | 8 (6.7) | 5 (3.9) | 7 (6.1) | 6 (4.4) | 13 (5.2) |
| Intraoperative blood transfusions | |||||
| N | 179 | 184 | 171 | 192 | 363 |
| Haemoglobin level (g/l), mean (SD), n | 82.5 (16.03), 121 | 84.5 (14.40), 117 | 83.0 (14.59), 120 | 84.0 (15.94), 118 | 83.5 (15.25), 238 |
| Professional making the decision to transfuse, n (%) | |||||
| Consultant | 108 (60.3) | 84 (45.7) | 91 (53.2) | 101 (52.6) | 192 (52.9) |
| Other doctor | 55 (30.7) | 57 (31.0) | 59 (34.5) | 53 (27.6) | 112 (30.9) |
| Other | 2 (1.1) | 12 (6.5) | 7 (4.1) | 7 (3.6) | 14 (3.9) |
| Missing | 14 (7.8) | 31 (16.8) | 14 (8.2) | 31 (16.2) | 45 (12.4) |
| Number of units transfused, n (%) | |||||
| One | 55 (30.7) | 60 (32.6) | 63 (36.8) | 52 (27.1) | 115 (31.7) |
| Two or more | 105 (58.7) | 101 (54.9) | 94 (55.0) | 112 (58.3) | 206 (56.7) |
| Missing | 19 (10.6) | 23 (12.5) | 14 (8.2) | 28 (14.6) | 42 (11.6) |
| Number of units transfused, mean (SD), n | 2.1 (1.45), 160 | 2.0 (1.29), 161 | 2.0 (1.45), 157 | 2.1 (1.30), 164 | 2.1 (1.37), 321 |
| Postoperative blood transfusions | |||||
| N | 1245 | 1315 | 1235 | 1325 | 2560 |
| Haemoglobin level (g/l), mean (SD), n | 79.7 (9.93), 1192 | 79.9 (9.24), 1207 | 80.0 (9.18), 1168 | 79.6 (9.97), 1231 | 79.8 (9.59), 2399 |
| Professional making the decision to transfuse, n (%) | |||||
| Nurse | 2 (0.2) | 6 (0.5) | 6 (0.5) | 2 (0.2) | 8 (0.3) |
| Consultant | 288 (23.1) | 376 (28.6) | 289 (23.4) | 375 (28.3) | 664 (25.9) |
| Other doctor | 781 (62.7) | 674 (51.3) | 731 (59.2) | 724 (54.6) | 1455 (56.8) |
| Other | 37 (3.0) | 42 (3.2) | 49 (4.0) | 30 (2.3) | 79 (3.1) |
| Missing | 137 (11.0) | 217 (16.5) | 160 (12.9) | 194 (14.7) | 354 (13.9) |
| Number of units transfused, n (%) | |||||
| One | 414 (33.3) | 353 (26.8) | 355 (28.7) | 412 (31.1) | 767 (30.0) |
| Two or more | 818 (65.7) | 940 (71.5) | 864 (70.0) | 894 (67.5) | 1758 (68.7) |
| Missing | 13 (1.0) | 22 (1.7) | 16 (1.3) | 19 (1.4) | 35 (1.4) |
| Number of units transfused, mean (SD), n | 1.8 (0.89), 1232 | 1.8 (0.69), 1293 | 1.8 (0.70), 1219 | 1.8 (0.86), 1306 | 1.8 (0.79), 2525 |
| Record of acute coronary ischaemia, n (%) | 95 (7.6) | 59 (4.5) | 79 (6.4) | 75 (5.7) | 154 (6.0) |
| Variable | Content | Follow-on | Total (N = 2222) | ||
|---|---|---|---|---|---|
| Standard (N = 1224) | Enhanced (N = 998) | Standard (N = 1118) | Enhanced (N = 1104) | ||
| Age (years), mean (SD), n | 75.3 (13.26), 1220 | 73.8 (14.35), 997 | 74.7 (13.56), 1118 | 74.6 (14.00), 1099 | 74.6 (13.78), 2217 |
| Gender (male), n (%) | 391 (31.9) | 317 (31.8) | 360 (32.2) | 348 (31.5) | 708 (31.9) |
| Surgical procedure, n (%) | |||||
| Orthopaedic | 470 (38.4) | 333 (33.4) | 411 (36.8) | 392 (35.5) | 803 (36.1) |
| Cardiac | 147 (12.0) | 189 (18.9) | 177 (15.8) | 159 (14.4) | 336 (15.1) |
| Fractured neck of femur | 424 (34.6) | 281 (28.2) | 346 (30.9) | 359 (32.5) | 705 (31.7) |
| Other | 182 (14.9) | 194 (19.4) | 184 (16.5) | 192 (17.4) | 376 (16.9) |
| Missing | 1 (0.1) | 1 (0.1) | 0 (0.0) | 2 (0.2) | 2 (0.1) |
| Attendance at preoperative clinic, n (%) | |||||
| Yes | 736 (60.1) | 657 (65.8) | 735 (65.7) | 658 (59.6) | 1393 (62.7) |
| No: orthopaedic | 52 (4.2) | 36 (3.6) | 26 (2.3) | 62 (5.6) | 88 (4.0) |
| No: cardiac | 15 (1.2) | 36 (3.6) | 25 (2.2) | 26 (2.4) | 51 (2.3) |
| No: fractured neck of femur | 385 (31.5) | 246 (24.6) | 306 (27.4) | 325 (29.4) | 631 (28.4) |
| No: other type of surgery | 35 (2.9) | 23 (2.3) | 26 (2.3) | 32 (2.9) | 58 (2.6) |
| No: surgery type missing | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Haemoglobin level at clinic (g/l), mean (SD), n | 121.8 (15.69), 482 | 119.4 (16.51), 372 | 121.1 (15.89), 435 | 120.4 (16.30), 419 | 120.8 (16.08), 854 |
| Haemoglobin level prior to surgery (g/l), mean (SD), n | 115.8 (16.86), 1189 | 115.9 (17.41), 972 | 116.8 (16.72), 1097 | 114.9 (17.45), 1064 | 115.9 (17.10), 2161 |
| Haemoglobin level on day 1 post surgery (g/l), mean (SD), n | 89.8 (13.78), 1069 | 91.1 (13.92), 879 | 90.9 (13.53), 989 | 89.8 (14.16), 959 | 90.4 (13.85), 1948 |
| Prescribed tranexamic acid, n (%) | 464 (37.9) | 426 (42.7) | 491 (43.9) | 399 (36.1) | 890 (40.1) |
| Patient died, n (%) | 32 (2.6) | 39 (3.9) | 35 (3.1) | 36 (3.3) | 71 (3.2) |
| Preoperative transfusion conducted, n (%) | 120 (9.8) | 127 (12.7) | 104 (9.3) | 143 (13.0) | 247 (11.1) |
| Intraoperative transfusion conducted, n (%) | 188 (15.4) | 185 (18.5) | 186 (16.6) | 187 (16.9) | 373 (16.8) |
| Postoperative transfusion conducted, n (%) | 1166 (95.3) | 938 (94.0) | 1059 (94.7) | 1045 (94.7) | 2104 (94.7) |
| Preoperative blood transfusions | |||||
| N | 120 | 127 | 104 | 143 | 247 |
| Haemoglobin level (g/l), mean (SD), n | 82.1 (13.52), 93 | 85.0 (15.21), 85 | 83.4 (15.05), 77 | 83.6 (13.94), 101 | 83.5 (14.39), 178 |
| Number of units transfused, n (%) | |||||
| One | 24 (20.0) | 22 (17.3) | 17 (16.3) | 29 (20.3) | 46 (18.6) |
| Two or more | 61 (50.8) | 62 (48.8) | 60 (57.7) | 63 (44.1) | 123 (49.8) |
| Missing | 35 (29.2) | 43 (33.9) | 27 (26.0) | 51 (35.7) | 78 (31.6) |
| Number of units transfused, mean (SD), n | 2.1 (1.16), 85 | 2.2 (2.12), 84 | 2.2 (2.19), 77 | 2.0 (1.15), 92 | 2.1 (1.71), 169 |
| Record of acute coronary ischaemia, n (%) | 8 (6.7) | 10 (7.9) | 6 (5.8) | 12 (8.4) | 18 (7.3) |
| Intraoperative blood transfusions | |||||
| N | 188 | 185 | 186 | 187 | 373 |
| Haemoglobin level (g/l), mean (SD), n | 83.3 (12.60), 112 | 83.0 (16.47), 105 | 83.0 (14.43), 112 | 83.2 (14.78), 105 | 83.1 (14.57), 217 |
| Number of units transfused, n (%) | |||||
| One | 72 (38.3) | 73 (39.5) | 71 (38.2) | 74 (39.6) | 145 (38.9) |
| Two or more | 116 (61.7) | 110 (59.5) | 114 (61.3) | 112 (59.9) | 226 (60.6) |
| Missing | 0 (0.0) | 2 (1.1) | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Number of units transfused, mean (SD), n | 2.1 (1.35), 188 | 2.1 (1.61), 183 | 2.2 (1.71), 185 | 2.0 (1.21), 186 | 2.1 (1.48), 371 |
| Postoperative blood transfusions | |||||
| N | 1166 | 938 | 1059 | 1045 | 2104 |
| Haemoglobin level (g/l), mean (SD), n | 79.5 (9.92), 1036 | 78.7 (9.57), 823 | 79.3 (9.78), 946 | 78.9 (9.77), 913 | 79.1 (9.77), 1859 |
| Number of units transfused, n (%) | |||||
| One | 481 (41.3) | 369 (39.3) | 421 (39.8) | 429 (41.1) | 850 (40.4) |
| Two or more | 645 (55.3) | 536 (57.1) | 602 (56.8) | 579 (55.4) | 1181 (56.1) |
| Missing | 40 (3.4) | 33 (3.5) | 36 (3.4) | 37 (3.5) | 73 (3.5) |
| Number of units transfused, mean (SD), n | 1.6 (0.66), 1126 | 1.7 (0.95), 905 | 1.7 (0.91), 1023 | 1.7 (0.68), 1008 | 1.7 (0.80), 2031 |
| Record of acute coronary ischaemia, n (%) | 61 (5.2) | 54 (5.8) | 54 (5.1) | 61 (5.8) | 115 (5.5) |
| Variable | Content | Follow-on | Total (N = 2714) | ||
|---|---|---|---|---|---|
| Standard (N = 1322) | Enhanced (N = 1392) | Standard (N = 1306) | Enhanced (N = 1408) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 209 (15.8) | 174 (12.5) | 177 (13.6) | 206 (14.6) | 383 (14.1) |
| Outside guidelines | 1036 (78.4) | 1097 (78.8) | 1050 (80.4) | 1083 (76.9) | 2133 (78.6) |
| Unclassified: ACI status unknown, haemoglobin level 70–80 g/l | 26 (2.0) | 15 (1.1) | 13 (1.0) | 28 (2.0) | 41 (1.5) |
| Unclassified: haemoglobin level missing | 51 (3.9) | 106 (7.6) | 66 (5.1) | 91 (6.5) | 157 (5.8) |
| Secondary outcome, mean (SD), n | |||||
| Total volume of blood transfused | 2.1 (1.40), 1287 | 2.2 (1.16), 1344 | 2.1 (1.22), 1275 | 2.2 (1.34), 1356 | 2.2 (1.28), 2631 |
| Supportive outcomes,a n (%) | |||||
| PBM standard 1 | |||||
| Meets standard | 215 (16.3) | 199 (14.3) | 181 (13.9) | 233 (16.5) | 414 (15.3) |
| Does not meet standard | 363 (27.5) | 345 (24.8) | 331 (25.3) | 377 (26.8) | 708 (26.1) |
| Insufficient information | 323 (24.4) | 430 (30.9) | 384 (29.4) | 369 (26.2) | 753 (27.7) |
| Excluded | 421 (31.8) | 418 (30.0) | 410 (31.4) | 429 (30.5) | 839 (30.9) |
| PBM standard 2 | |||||
| Meets standard | 8 (0.6) | 15 (1.1) | 11 (0.8) | 12 (0.9) | 23 (0.8) |
| Does not meet standard | 99 (7.5) | 96 (6.9) | 86 (6.6) | 109 (7.7) | 195 (7.2) |
| Insufficient information | 4 (0.3) | 13 (0.9) | 9 (0.7) | 8 (0.6) | 17 (0.6) |
| Excluded | 1211 (91.6) | 1268 (91.1) | 1200 (91.9) | 1279 (90.8) | 2479 (91.3) |
| PBM standard 3 | |||||
| Meets standard | 2 (0.2) | 1 (0.1) | 3 (0.2) | 0 (0.0) | 3 (0.1) |
| Does not meet standard | 25 (1.9) | 34 (2.4) | 24 (1.8) | 35 (2.5) | 59 (2.2) |
| Insufficient information | 27 (2.0) | 35 (2.5) | 29 (2.2) | 33 (2.3) | 62 (2.3) |
| Excluded | 1268 (95.9) | 1322 (95.0) | 1250 (95.7) | 1340 (95.2) | 2590 (95.4) |
| PBM standard 4 | |||||
| Meets standard | 30 (2.3) | 34 (2.4) | 21 (1.6) | 43 (3.1) | 64 (2.4) |
| Does not meet standard | 78 (5.9) | 82 (5.9) | 79 (6.0) | 81 (5.8) | 160 (5.9) |
| Insufficient information | 3 (0.2) | 8 (0.6) | 6 (0.5) | 5 (0.4) | 11 (0.4) |
| Excluded | 1211 (91.6) | 1268 (91.1) | 1200 (91.9) | 1279 (90.8) | 2479 (91.3) |
| PBM standard 8 | |||||
| Meets standard | 57 (4.3) | 38 (2.7) | 47 (3.6) | 48 (3.4) | 95 (3.5) |
| Does not meet standard | 827 (62.6) | 906 (65.1) | 888 (68.0) | 845 (60.0) | 1733 (63.9) |
| Insufficient information | 361 (27.3) | 371 (26.7) | 300 (23.0) | 432 (30.7) | 732 (27.0) |
| Excluded | 77 (5.8) | 77 (5.5) | 71 (5.4) | 83 (5.9) | 154 (5.7) |
| Intermediate outcomes, n (%) | |||||
| Planned surgery date equals actual surgery date | 642 (48.6) | 651 (46.8) | 629 (48.2) | 664 (47.2) | 1293 (47.6) |
| Attendance at preoperative assessment clinic | 839 (63.5) | 922 (66.2) | 841 (64.4) | 920 (65.3) | 1761 (64.9) |
| Ferritin checked | 68 (5.1) | 79 (5.7) | 52 (4.0) | 95 (6.7) | 147 (5.4) |
| Oral iron before operation | 135 (10.2) | 152 (10.9) | 141 (10.8) | 146 (10.4) | 287 (10.6) |
| i.v. iron before operation | 12 (0.9) | 8 (0.6) | 10 (0.8) | 10 (0.7) | 20 (0.7) |
| Prescribed tranexamic acid | 454 (34.3) | 440 (31.6) | 425 (32.5) | 469 (33.3) | 894 (32.9) |
| Collection for IOCS commenced | 162 (12.3) | 139 (10.0) | 166 (12.7) | 135 (9.6) | 301 (11.1) |
| Postoperative cell salvage used | 24 (1.8) | 12 (0.9) | 19 (1.5) | 17 (1.2) | 36 (1.3) |
| Patient given postoperative iron | 195 (14.8) | 251 (18.0) | 239 (18.3) | 207 (14.7) | 446 (16.4) |
| Length of postoperative hospital stay, mean (SD), n | 12.7 (11.63), 1299 | 13.1 (11.72), 1359 | 13.1 (12.48), 1288 | 12.7 (10.87), 1370 | 12.9 (11.67), 2658 |
| Preoperative blood transfusions | |||||
| N | 120 | 129 | 114 | 135 | 249 |
| Supportive outcomes, n (%) | |||||
| Preoperative component of primary outcome | |||||
| Acceptable | 11 (9.2) | 18 (14.0) | 13 (11.4) | 16 (11.9) | 29 (11.6) |
| Outside guidelines | 102 (85.0) | 102 (79.1) | 96 (84.2) | 108 (80.0) | 204 (81.9) |
| Unclassified: ACI status unknown, haemoglobin level 70–80 g/l | 2 (1.7) | 3 (2.3) | 1 (0.9) | 4 (3.0) | 5 (2.0) |
| Unclassified: haemoglobin level missing | 5 (4.2) | 6 (4.7) | 4 (3.5) | 7 (5.2) | 11 (4.4) |
| Number of units transfused preoperatively | |||||
| One | 26 (21.7) | 26 (20.2) | 19 (16.7) | 33 (24.4) | 52 (20.9) |
| Two or more | 93 (77.5) | 101 (78.3) | 94 (82.5) | 100 (74.1) | 194 (77.9) |
| Missing | 1 (0.8) | 2 (1.6) | 1 (0.9) | 2 (1.5) | 3 (1.2) |
| Postoperative blood transfusions | |||||
| N | 1245 | 1315 | 1235 | 1325 | 2560 |
| Supportive outcomes, n (%) | |||||
| Postoperative component of primary outcome | |||||
| Acceptable | 205 (16.5) | 167 (12.7) | 173 (14.0) | 199 (15.0) | 372 (14.5) |
| Outside guidelines | 964 (77.4) | 1027 (78.1) | 985 (79.8) | 1006 (75.9) | 1991 (77.8) |
| Unclassified: ACI status unknown, haemoglobin level 70–80 g/l | 25 (2.0) | 16 (1.2) | 13 (1.1) | 28 (2.1) | 41 (1.6) |
| Unclassified: haemoglobin level missing | 51 (4.1) | 105 (8.0) | 64 (5.2) | 92 (6.9) | 156 (6.1) |
| Number of units transfused postoperatively | |||||
| One | 414 (33.3) | 353 (26.8) | 355 (28.7) | 412 (31.1) | 767 (30.0) |
| Two or more | 818 (65.7) | 940 (71.5) | 864 (70.0) | 894 (67.5) | 1758 (68.7) |
| Missing | 13 (1.0) | 22 (1.7) | 16 (1.3) | 19 (1.4) | 35 (1.4) |
| Variable | Content | Follow-on | Total (N = 2222) | ||
|---|---|---|---|---|---|
| Standard (N = 1224) | Enhanced (N = 998) | Standard (N = 1118) | Enhanced (N = 1104) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 198 (16.2) | 152 (15.2) | 176 (15.7) | 174 (15.8) | 350 (15.8) |
| Outside guidelines | 901 (73.6) | 726 (72.7) | 822 (73.5) | 805 (72.9) | 1627 (73.2) |
| Unclassified: ACI status unknown, haemoglobin level 70–80 g/l | 1 (0.1) | 2 (0.2) | 3 (0.3) | 0 (0.0) | 3 (0.1) |
| Unclassified: haemoglobin level missing | 124 (10.1) | 118 (11.8) | 117 (10.5) | 125 (11.3) | 242 (10.9) |
| Secondary outcome, mean (SD), n | |||||
| Total volume of blood transfused | 2.0 (1.22), 1147 | 2.2 (1.71), 921 | 2.1 (1.62), 1052 | 2.1 (1.28), 1016 | 2.1 (1.46), 2068 |
| Supportive outcomes,a n (%) | |||||
| PBM standard 1 | |||||
| Meets standard | 240 (19.6) | 191 (19.1) | 218 (19.5) | 213 (19.3) | 431 (19.4) |
| Does not meet standard | 332 (27.1) | 265 (26.6) | 285 (25.5) | 312 (28.3) | 597 (26.9) |
| Insufficient information | 228 (18.6) | 261 (26.2) | 269 (24.1) | 220 (19.9) | 489 (22.0) |
| Excluded | 424 (34.6) | 281 (28.2) | 346 (30.9) | 359 (32.5) | 705 (31.7) |
| PBM standard 2 | |||||
| Meets standard | 14 (1.1) | 9 (0.9) | 12 (1.1) | 11 (1.0) | 23 (1.0) |
| Does not meet standard | 67 (5.5) | 68 (6.8) | 63 (5.6) | 72 (6.5) | 135 (6.1) |
| Insufficient information | 35 (2.9) | 46 (4.6) | 28 (2.5) | 53 (4.8) | 81 (3.6) |
| Excluded | 1108 (90.5) | 875 (87.7) | 1015 (90.8) | 968 (87.7) | 1983 (89.2) |
| PBM standard 3 | |||||
| Meets standard | 2 (0.2) | 1 (0.1) | 0 (0.0) | 3 (0.3) | 3 (0.1) |
| Does not meet standard | 24 (2.0) | 23 (2.3) | 26 (2.3) | 21 (1.9) | 47 (2.1) |
| Insufficient information | 48 (3.9) | 57 (5.7) | 39 (3.5) | 66 (6.0) | 105 (4.7) |
| Excluded | 1150 (94.0) | 917 (91.9) | 1053 (94.2) | 1014 (91.8) | 2067 (93.0) |
| PBM standard 4 | |||||
| Meets standard | 29 (2.4) | 26 (2.6) | 21 (1.9) | 34 (3.1) | 55 (2.5) |
| Does not meet standard | 51 (4.2) | 48 (4.8) | 52 (4.7) | 47 (4.3) | 99 (4.5) |
| Insufficient information | 36 (2.9) | 49 (4.9) | 30 (2.7) | 55 (5.0) | 85 (3.8) |
| Excluded | 1108 (90.5) | 875 (87.7) | 1015 (90.8) | 968 (87.7) | 1983 (89.2) |
| PBM standard 8 | |||||
| Meets standard | 68 (5.6) | 32 (3.2) | 48 (4.3) | 52 (4.7) | 100 (4.5) |
| Does not meet standard | 717 (58.6) | 546 (54.7) | 621 (55.5) | 642 (58.2) | 1263 (56.8) |
| Insufficient information | 381 (31.1) | 360 (36.1) | 390 (34.9) | 351 (31.8) | 741 (33.3) |
| Excluded | 58 (4.7) | 60 (6.0) | 59 (5.3) | 59 (5.3) | 118 (5.3) |
| Intermediate outcomes, n (%) | |||||
| Planned surgery date equals actual surgery date | 813 (66.4) | 547 (54.8) | 743 (66.5) | 617 (55.9) | 1360 (61.2) |
| Attendance at preoperative assessment clinic | 736 (60.1) | 657 (65.8) | 735 (65.7) | 658 (59.6) | 1393 (62.7) |
| Ferritin checked | 71 (5.8) | 76 (7.6) | 68 (6.1) | 79 (7.2) | 147 (6.6) |
| Oral iron before operation | 144 (11.8) | 106 (10.6) | 131 (11.7) | 119 (10.8) | 250 (11.3) |
| i.v. iron before operation | 15 (1.2) | 12 (1.2) | 9 (0.8) | 18 (1.6) | 27 (1.2) |
| Prescribed tranexamic acid | 464 (37.9) | 426 (42.7) | 491 (43.9) | 399 (36.1) | 890 (40.1) |
| Collection for IOCS commenced | 127 (10.4) | 125 (12.5) | 132 (11.8) | 120 (10.9) | 252 (11.3) |
| Postoperative cell salvage used | 15 (1.2) | 19 (1.9) | 20 (1.8) | 14 (1.3) | 34 (1.5) |
| Patient given postoperative iron | 185 (15.1) | 169 (16.9) | 193 (17.3) | 161 (14.6) | 354 (15.9) |
| Length of postoperative hospital stay, mean (SD), n | 12.5 (10.41), 1195 | 12.8 (12.15), 979 | 12.2 (10.88), 1106 | 13.1 (11.56), 1068 | 12.7 (11.22), 2174 |
| Preoperative blood transfusions | |||||
| N | 120 | 127 | 104 | 143 | 247 |
| Supportive outcomes, n (%) | |||||
| Preoperative component of primary outcome | |||||
| Acceptable | 16 (13.3) | 10 (7.9) | 12 (11.5) | 14 (9.8) | 26 (10.5) |
| Outside guidelines | 77 (64.2) | 75 (59.1) | 65 (62.5) | 87 (60.8) | 152 (61.5) |
| Unclassified: haemoglobin level missing | 27 (22.5) | 42 (33.1) | 27 (26.0) | 42 (29.4) | 69 (27.9) |
| Number of units transfused preoperatively | |||||
| One | 24 (20.0) | 22 (17.3) | 17 (16.3) | 29 (20.3) | 46 (18.6) |
| Two or more | 61 (50.8) | 62 (48.8) | 60 (57.7) | 63 (44.1) | 123 (49.8) |
| Missing | 35 (29.2) | 43 (33.9) | 27 (26.0) | 51 (35.7) | 78 (31.6) |
| Postoperative blood transfusions | |||||
| N | 1166 | 938 | 1059 | 1045 | 2104 |
| Supportive outcomes, n (%) | |||||
| Postoperative component of primary outcome | |||||
| Acceptable | 198 (17.0) | 155 (16.5) | 174 (16.4) | 179 (17.1) | 353 (16.8) |
| Outside guidelines | 844 (72.4) | 671 (71.5) | 774 (73.1) | 741 (70.9) | 1515 (72.0) |
| Unclassified: ACI status unknown, haemoglobin level 70–80 g/l | 1 (0.1) | 2 (0.2) | 3 (0.3) | 0 (0.0) | 3 (0.1) |
| Unclassified: haemoglobin level missing | 123 (10.5) | 110 (11.7) | 108 (10.2) | 125 (12.0) | 233 (11.1) |
| Number of units transfused postoperatively | |||||
| One | 481 (41.3) | 369 (39.3) | 421 (39.8) | 429 (41.1) | 850 (40.4) |
| Two or more | 645 (55.3) | 536 (57.1) | 602 (56.8) | 579 (55.4) | 1181 (56.1) |
| Missing | 40 (3.4) | 33 (3.5) | 36 (3.4) | 37 (3.5) | 73 (3.5) |
| Analysis | Unadjusted proportion acceptable | Estimated adjusted risk difference (95% CI) | Estimated adjusted odds ratio (97.5% CI) | Estimated adjusted odds ratio (95% CI) | p-value | n | |
|---|---|---|---|---|---|---|---|
| Standard | Enhanced | ||||||
| Primary analysis (multiple imputation, 100 imputations, full imputation model) | |||||||
| Content | 0.184 | 0.176 | –0.01 (–0.07 to 0.04) | 0.91 (0.61 to 1.36) | 0.91 (0.64 to 1.30) | 0.605 | 2222 |
| Follow-on | 0.181 | 0.180 | 0.01 (–0.05 to 0.06) | 1.05 (0.68 to 1.61) | 1.05 (0.72 to 1.52) | 0.807 | 2222 |
| Interaction | 0.184 | 0.167 | 0.05 (–0.08 to 0.13) | 1.15 (0.52 to 2.56) | 1.15 (0.57 to 2.31) | 0.696 | 2222 |
| Sensitivity analyses | |||||||
| Complete-case analysis | |||||||
| Content | 0.180 | 0.173 | –0.01 (–0.06 to 0.04) | 0.93 (0.62 to 1.40) | 0.93 (0.65 to 1.33) | 0.694 | 1977 |
| Follow-on | 0.176 | 0.178 | 0.00 (–0.05 to 0.06) | 1.02 (0.68 to 1.53) | 1.02 (0.71 to 1.45) | 0.934 | 1977 |
| Interaction | 0.181 | 0.163 | 0.00 (–0.10 to 0.10) | 0.98 (0.45 to 2.13) | 0.98 (0.50 to 1.93) | 0.947 | 1977 |
| Analysis | Unadjusted proportion acceptable | Estimated adjusted risk difference (95% CI) | p-value | n | |
|---|---|---|---|---|---|
| Standard | Enhanced | ||||
| Preoperative component of the primary outcomea | |||||
| Content | 0.154 | 0.117 | 0.68 (0.28 to 1.67) | 0.398 | 247 |
| Follow-on | 0.145 | 0.128 | 0.85 (0.36 to 2.00) | 0.712 | 247 |
| Interaction | 0.139 | 0.122 | 1.92 (0.35 to 10.67) | 0.456 | 247 |
| Postoperative component of the primary outcome | |||||
| Content | 0.195 | 0.191 | 0.94 (0.66 to 1.34) | 0.734 | 2104 |
| Follow-on | 0.190 | 0.196 | 1.09 (0.74 to 1.59) | 0.673 | 2104 |
| Interaction | 0.195 | 0.185 | 1.12 (0.55 to 2.27) | 0.764 | 2104 |
| PBM standard 1a,b | |||||
| Content | 0.390 | 0.364 | 0.96 (0.69 to 1.35) | 0.828 | 1517 |
| Follow-on | 0.382 | 0.374 | 0.96 (0.68 to 1.34) | 0.793 | 1517 |
| Interaction | 0.379 | 0.375 | 1.18 (0.61 to 2.29) | 0.625 | 1517 |
| PBM standard 2a | |||||
| Content | 0.129 | 0.138 | 1.01 (0.33 to 3.14) | 0.983 | 239 |
| Follow-on | 0.179 | 0.098 | 0.54 (0.22 to 1.31) | 0.174 | 239 |
| Interaction | 0.146 | 0.097 | 1.03 (0.17 to 6.18) | 0.972 | 239 |
| PBM standard 4a | |||||
| Content | 0.515 | 0.580 | 1.86 (0.89 to 3.86) | 0.098 | 239 |
| Follow-on | 0.476 | 0.604 | 1.66 (0.82 to 3.37) | 0.159 | 239 |
| Interaction | 0.531 | 0.600 | 0.42 (0.10 to 1.71) | 0.226 | 239 |
| PBM standard 8a | |||||
| Content | 0.114 | 0.078 | 0.55 (0.23 to 1.30) | 0.170 | 2104 |
| Follow-on | 0.099 | 0.097 | 0.86 (0.35 to 2.11) | 0.746 | 2104 |
| Interaction | 0.108 | 0.060 | 0.51 (0.11 to 2.41) | 0.396 | 2104 |
| Variable | Content | Follow-on | Total (N = 135) | ||
|---|---|---|---|---|---|
| Standard (N = 66) | Enhanced (N = 69) | Standard (N = 67) | Enhanced (N = 68) | ||
| Total number of incidents, median (IQR) (range) | |||||
| Baseline | 14.5 (5–22) (0–154) | 13 (9–24) (3–77) | 13 (8–22) (0–77) | 16 (7–24) (0–154) | 14 (8–23) (0–154) |
| Follow-up | 13 (6–21) (1–158) | 13 (7–22) (0–68) | 12 (5–21) (0–68) | 13 (7.5–23) (1–158) | 13 (6–22) (0–158) |
| October–December 2014 (baseline) | 3 (1–6) (0–27) | 3 (1–6) (0–21) | 3 (1–6) (0–21) | 3 (1–6) (0–27) | 3 (1–6) (0–27) |
| January–March 2015 (baseline) | 4 (1–7) (0–40) | 4 (2–8) (0–28) | 3 (2–7) (0–28) | 4 (1–7.5) (0–40) | 4 (1–7) (0–40) |
| April–June 2015 (baseline) | 3 (1–6) (0–40) | 4 (2–5) (0–21) | 3 (2–5) (0–26) | 4 (2–7) (0–40) | 3 (2–6) (0–40) |
| July–September 2015 (baseline) | 4 (1–7) (0–47) | 4 (2–6) (0–24) | 3 (1–5) (0–24) | 4 (1.5–7) (0–47) | 4 (1–7) (0–47) |
| November 2015–January 2016 (follow-up) | 3 (1–6) (0–49) | 3 (1–5) (0–18) | 3 (1–6) (0–18) | 3 (1–5) (0–49) | 3 (1–6) (0–49) |
| February–April 2016 (follow-up) | 3 (1–6) (0–37) | 3 (1–5) (0–15) | 3 (1–5) (0–15) | 3 (2–6.5) (0–37) | 3 (1–6) (0–37) |
| May–July 2016 (follow-up) | 3 (1–6) (0–30) | 3 (2–6) (0–24) | 3 (1–6) (0–24) | 3.5 (1–6) (0–30) | 3 (1–6) (0–30) |
| August–October 2016 (follow-up) | 3 (1–6) (0–42) | 3 (1–7) (0–25) | 3 (1–6) (0–19) | 3 (1.5–7) (0–42) | 3 (1–7) (0–42) |
| Number of relevant errors, median (IQR) (range) | |||||
| Baseline | 0 (0–1) (0–6) | 0 (0–1) (0–5) | 0 (0–1) (0–4) | 0 (0–1) (0–6) | 0 (0–1) (0–6) |
| Follow-up | 0 (0–1) (0–9) | 0 (0–1) (0–6) | 0 (0–1) (0–6) | 0 (0–1) (0–9) | 0 (0–1) (0–9) |
| October–December 2014 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) |
| January–March 2015 (baseline) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| April–June 2015 (baseline) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| July–September 2015 (baseline) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) |
| November 2015–January 2016 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| February–April 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| May–July 2016 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| August–October 2016 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| Number of relevant near-miss or ‘right blood right patient’ incidents, median (IQR) (range) | |||||
| Baseline | 0 (0–1) (0–2) | 0 (0–0) (0–2) | 0 (0–1) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) |
| Follow-up | 0 (0–1) (0–9) | 0 (0–0) (0–4) | 0 (0–1) (0–6) | 0 (0–0) (0–9) | 0 (0–0) (0–9) |
| October–December 2014 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| January–March 2015 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| April–June 2015 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| July–September 2015 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) |
| November 2015–January 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| February–April 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–1) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) |
| May–July 2016 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| August–October 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| Total number of unpredictable incidents, median (IQR) (range) | |||||
| Baseline | 1 (0–3) (0–10) | 1 (0–2) (0–15) | 1 (0–3) (0–15) | 1 (0–2) (0–14) | 1 (0–3) (0–15) |
| Follow-up | 1 (0–2) (0–8) | 1 (0–2) (0–8) | 1 (0–2) (0–7) | 1 (0–2) (0–8) | 1 (0–2) (0–8) |
| October–December 2014 (baseline) | 0 (0–1) (0–5) | 0 (0–0) (0–4) | 0 (0–1) (0–5) | 0 (0–1) (0–4) | 0 (0–1) (0–5) |
| January–March 2015 (baseline) | 0 (0–1) (0–4) | 0 (0–1) (0–5) | 0 (0–1) (0–5) | 0 (0–1) (0–4) | 0 (0–1) (0–5) |
| April–June 2015 (baseline) | 0 (0–1) (0–4) | 0 (0–1) (0–5) | 0 (0–1) (0–5) | 0 (0–1) (0–5) | 0 (0–1) (0–5) |
| July–September 2015 (baseline) | 0 (0–0) (0–5) | 0 (0–1) (0–5) | 0 (0–1) (0–4) | 0 (0–1) (0–5) | 0 (0–1) (0–5) |
| November 2015–January 2016 (follow-up) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–1) (0–2) | 0 (0–1) (0–3) |
| February–April 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–5) | 0 (0–1) (0–2) | 0 (0–0) (0–5) | 0 (0–0) (0–5) |
| May–July 2016 (follow-up) | 0 (0–0) (0–4) | 0 (0–1) (0–4) | 0 (0–0) (0–3) | 0 (0–1) (0–4) | 0 (0–0) (0–4) |
| August–October 2016 (follow-up) | 0 (0–1) (0–3) | 0 (0–0) (0–4) | 0 (0–1) (0–3) | 0 (0–1) (0–4) | 0 (0–1) (0–4) |
| Total number of possibly preventable incidents, median (IQR) (range) | |||||
| Baseline | 0 (0–2) (0–72) | 1 (0–2) (0–9) | 1 (0–2) (0–9) | 1 (0–2) (0–72) | 1 (0–2) (0–72) |
| Follow-up | 0 (0–1) (0–17) | 0 (0–1) (0–7) | 0 (0–1) (0–7) | 0 (0–1) (0–17) | 0 (0–1) (0–17) |
| October–December 2014 (baseline) | 0 (0–0) (0–11) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–0) (0–11) | 0 (0–1) (0–11) |
| January–March 2015 (baseline) | 0 (0–0) (0–22) | 0 (0–0) (0–4) | 0 (0–0) (0–5) | 0 (0–1) (0–22) | 0 (0–0) (0–22) |
| April–June 2015 (baseline) | 0 (0–1) (0–21) | 0 (0–1) (0–4) | 0 (0–1) (0–4) | 0 (0–1) (0–21) | 0 (0–1) (0–21) |
| July–September 2015 (baseline) | 0 (0–0) (0–18) | 0 (0–0) (0–5) | 0 (0–0) (0–4) | 0 (0–0) (0–18) | 0 (0–0) (0–18) |
| November 2015–January 2016 (follow-up) | 0 (0–0) (0–12) | 0 (0–0) (0–3) | 0 (0–0) (0–6) | 0 (0–0) (0–12) | 0 (0–0) (0–12) |
| February–April 2016 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) |
| May–July 2016 (follow-up) | 0 (0–0) (0–4) | 0 (0–0) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–4) | 0 (0–0) (0–4) |
| August–October 2016 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–4) | 0 (0–0) (0–4) | 0 (0–0) (0–2) | 0 (0–0) (0–4) |
| Variable | Content | Follow-on | Total (N = 135) | ||
|---|---|---|---|---|---|
| Standard (N = 66) | Enhanced (N = 69) | Standard (N = 67) | Enhanced (N = 68) | ||
| Total volume of RBCs transfused (units), median (IQR) (range), n | |||||
| Baseline | 8048.5 (5595–10,746) (138–31,311), 62 | 8172 (5175–11,863) (548–39,006), 66 | 7346.5 (5160.5–11,373.5) (548–31,311), 64 | 8194 (5917.5–11,228.5) (138–39,006), 64 | 8147 (5372–11,245.5) (138–39,006), 128 |
| Follow-up | 6736.5 (4920–10,316) (565–30,230), 62 | 7506 (5072–12,756) (649–32,670), 67 | 6670 (4920–10,534) (2240–29,528), 63 | 7438 (5085–11,059) (565–32,670), 66 | 7012 (4970–10,927) (565–32,670), 129 |
| October–December 2014 (baseline) | 2040 (1427–2802) (235–7749), 61 | 2037 (1340–3059) (574–9132), 65 | 1909 (1222–2996) (594–8008), 63 | 2082 (1476–2853) (235–9132), 63 | 2039 (1340–2946) (235–9132), 126 |
| January–March 2015 (baseline) | 1936 (1421–2798) (168–7909), 61 | 1895 (1322–3158) (509–9284), 65 | 1798 (1240–3002) (648–7909), 63 | 1936 (1466–2798) (168–9284), 63 | 1922 (1341–2905) (168–9284), 126 |
| April–June 2015 (baseline) | 2009 (1342–2762) (195–7998), 61 | 1989.5 (1298–2924) (413–9986), 66 | 1756.5 (1247.5–2801) (488–7998), 64 | 2109 (1427–2858) (195–9986), 63 | 2009 (1298–2813) (195–9986), 127 |
| July–September 2015 (baseline) | 1993 (1359–2576) (138–7655), 61 | 1917 (1371–2878) (433–10,604), 65 | 1921 (1359–2771) (712–7655), 62 | 1955 (1378.5–2867.5) (138–10,604), 64 | 1943 (1367–2793) (138–10,604), 126 |
| November 2015–January 2016 (follow-up) | 1828.5 (1278.5–2578) (154–7815), 60 | 2083 (1252–3004) (400–9488), 66 | 1798.5 (1243–2813) (400–7815), 62 | 2027 (1330–2751) (154–9488), 64 | 1910 (1252–2755) (154–9488), 126 |
| February–April 2016 (follow-up) | 1710.5 (1246.5–2506.5) (129–7458), 60 | 1999.5 (1231–3080) (644–9990), 66 | 1772 (1208–2610) (724–7458), 63 | 1896 (1281–2779) (129–9990), 63 | 1811 (1231–2710) (129–9990), 126 |
| May–July 2016 (follow-up) | 1663 (1155–2471) (158–8124), 61 | 2077 (1299–3535) (400–7684), 65 | 1713 (1235–2838) (400–7684), 62 | 2051.5 (1269–3015) (158–8124), 64 | 1836.5 (1246–2993) (158–8124), 126 |
| August–October 2016 (follow-up) | 1644.5 (1090–2716) (100–7792), 62 | 1820 (1176–3200) (571–6819), 66 | 1648 (1120–2725) (415–7066), 63 | 1887 (1233–2975) (100–7792), 65 | 1718 (1147.5–2897.5) (100–7792), 128 |
| Total gross volume of RBCs issued, median (IQR) (range), n | |||||
| Baseline | 7991 (5101–11,185) (659–30,843), 63 | 8302 (5296–13,375.5) (3126–41,681), 68 | 7809 (5070–11,460) (3102–31,085), 65 | 8221.5 (5439–12,100) (659–41,681), 66 | 8113 (5215–11,525) (659–41,681), 131 |
| Follow-up | 8678 (5640–11,365) (828–31,827), 63 | 8743.5 (5456–13,959.5) (3296–41,827), 68 | 8678 (5455–11,828) (3407–32,605), 65 | 8709 (6048–11,711) (828–41,827), 66 | 8687 (5551–11,828) (828–41,827), 131 |
| October–December 2014 (baseline) | 2209 (1459–2865) (248–7876), 63 | 2144 (1366.5–3513) (786–10,176), 68 | 2209 (1380–2955) (806–8222), 65 | 2159 (1493–3156) (248–10,176), 66 | 2209 (1400–2980) (248–10,176), 131 |
| January–March 2015 (baseline) | 2139 (1399–2775) (190–8022), 63 | 2132.5 (1402–3346) (748–9798), 68 | 2131 (1365–3012) (920–8509), 65 | 2153.5 (1491–2887) (190–9798), 66 | 2134 (1399–2987) (190–9798), 131 |
| April–June 2015 (baseline) | 2062 (1343–2884) (213–8118), 63 | 2112.5 (1374–3274) (907–10,840), 68 | 2019 (1332–2946) (792–8118), 65 | 2189 (1411–2939) (213–10,840), 66 | 2081 (1355–2946) (213–10,840), 131 |
| July–September 2015 (baseline) | 2134 (1400–2869) (177–7811), 63 | 2205.5 (1408.5–3532.5) (738–11,013), 68 | 2126 (1399–2875) (738–8126), 65 | 2182 (1465–2987) (177–11,013), 66 | 2138 (1401–2965) (177–11,013), 131 |
| November 2015–January 2016 (follow-up) | 2018 (1346–2775) (188–7954), 63 | 2122.5 (1327–3256) (778–9868), 68 | 2012 (1276–2921) (756–7954), 65 | 2140 (1432–2888) (188–9868), 66 | 2097 (1327–2921) (188–9868), 131 |
| February–April 2016 (follow-up) | 1902 (1368–2698) (154–7589), 63 | 2107 (1337.5–3302) (741–10,277), 68 | 1876 (1272–2745) (753–7819), 65 | 2043.5 (1370–3050) (154–10,277), 66 | 2043 (1357–2971) (154–10,277), 131 |
| May–July 2016 (follow-up) | 2018 (1223–2723) (173–8286), 63 | 2130 (1298.5–3460.5) (765–10,506), 68 | 1996 (1278–2821) (749–7956), 65 | 2118 (1426–3028) (173–10,506), 66 | 2030 (1288–3008) (173–10,506), 131 |
| August–October 2016 (follow-up) | 2008 (1192–2699) (144–7948), 63 | 2014 (1266–3360.5) (780–11,030), 68 | 1828 (1194–2747) (723–7583), 65 | 2021.5 (1350–2986) (144–11,030), 66 | 2008 (1240–2852) (144–11,030), 131 |
| Total volume of RBCs wasted, median (IQR) (range), n | |||||
| Baseline | 162.5 (107–265) (2–1171), 62 | 165 (98–294) (22–1978), 66 | 161 (99.5–256) (21–1565), 64 | 169.5 (105–292) (2–1978), 64 | 163 (100.5–279) (2–1978), 128 |
| Follow-up | 157 (94–280) (3–1212), 62 | 182 (88–357) (23–1326), 67 | 155 (88–282) (34–1282), 63 | 206 (94–337) (3–1326), 66 | 162 (91–325) (3–1326), 129 |
| October–December 2014 (baseline) | 41 (26–71) (3–303), 61 | 39 (21–78) (4–655), 65 | 41 (21–71) (3–415), 63 | 39 (26–78) (4–655), 63 | 40 (23–76) (3–655), 126 |
| January–March 2015 (baseline) | 36 (25–61) (2–266), 61 | 43 (23–80) (5–514), 65 | 37 (25–66) (2–442), 63 | 43 (22–79) (7–514), 63 | 37 (23–67) (2–514), 126 |
| April–June 2015 (baseline) | 40 (25–64) (3–269), 61 | 42 (25–80) (4–421), 66 | 38.5 (22–71) (3–406), 64 | 42 (25–81) (4–421), 63 | 42 (25–73) (3–421), 127 |
| July–September 2015 (baseline) | 44 (28–70) (2–333), 61 | 40 (28–75) (2–409), 65 | 38 (27–64) (7–333), 62 | 44 (28–79) (2–409), 64 | 43 (28–75) (2–409), 126 |
| November 2015–January 2016 (follow-up) | 36 (22.5–74.5) (1–236), 60 | 47 (24–76) (2–380), 66 | 35 (22–68) (2–308), 62 | 46.5 (23.5–80.5) (1–380), 64 | 42 (23–75) (1–380), 126 |
| February–April 2016 (follow-up) | 45 (27–75.5) (0–254), 60 | 46.5 (25–99) (6–354), 66 | 39 (26–78) (6–254), 63 | 53 (25–99) (0–354), 63 | 46 (26–81) (0–354), 126 |
| May–July 2016 (follow-up) | 41 (21–71) (1–544), 61 | 47 (23–88) (9–389), 65 | 38.5 (23–79) (9–389), 62 | 49.5 (23–79.5) (1–544), 64 | 43 (23–79) (1–544), 126 |
| August–October 2016 (follow-up) | 37 (20–75) (1–371), 62 | 47.5 (20–92) (4–351), 66 | 36 (20–75) (4–365), 63 | 48 (20–82) (1–371), 65 | 42.5 (20–82) (1–371), 128 |
| Variable | Content | Follow-on | Total (N = 4439) | ||
|---|---|---|---|---|---|
| Standard (N = 2228) | Enhanced (N = 2211) | Standard (N = 2188) | Enhanced (N = 2251) | ||
| Age (years), median (IQR), n | 73.0 (64.0–80.0), 2227 | 72.0 (64.0–80.0), 2208 | 72.0 (64.0–80.0), 2187 | 72.0 (64.0–80.0), 2248 | 72.0 (64.0–80.0), 4435 |
| Gender (male), n (%) | 1306 (58.6) | 1335 (60.4) | 1301 (59.5) | 1340 (59.5) | 2641 (59.5) |
| Haematological diagnosis, n (%) | |||||
| Acute leukaemia | 469 (21.1) | 460 (20.8) | 435 (19.9) | 494 (21.9) | 929 (20.9) |
| Chronic leukaemia/lymphoma and myeloma | 751 (33.7) | 752 (34.0) | 754 (34.5) | 749 (33.3) | 1503 (33.9) |
| MDS and aplastic anaemia | 1038 (46.6) | 975 (44.1) | 1006 (46.0) | 1007 (44.7) | 2013 (45.3) |
| Additional treatment for haematological diagnosis, n (%) | 723 (32.5) | 615 (27.8) | 617 (28.2) | 721 (32.0) | 1338 (30.1) |
| Stem cell transplant | 128 (5.7) | 126 (5.7) | 94 (4.3) | 160 (7.1) | 254 (5.7) |
| Intensive chemotherapy | 574 (79.4) | 461 (75.0) | 494 (80.1) | 541 (75.0) | 1035 (77.4) |
| Participating in clinical study | 191 (8.6) | 166 (7.5) | 195 (8.9) | 162 (7.2) | 357 (8.0) |
| Transfusion type, n (%) | |||||
| RBCs and platelets | 744 (33.4) | 643 (29.1) | 683 (31.2) | 704 (31.3) | 1387 (31.2) |
| RBCs only | 1360 (61.0) | 1421 (64.3) | 1377 (62.9) | 1404 (62.4) | 2781 (62.6) |
| Platelets only | 124 (5.6) | 147 (6.6) | 128 (5.9) | 143 (6.4) | 271 (6.1) |
| RBC transfusions | |||||
| N | 2104 | 2064 | 2060 | 2108 | 4168 |
| Number of units transfused, mean (SD), n | 1.9 (0.56), 2098 | 2.0 (0.59), 2055 | 1.9 (0.58), 2051 | 2.0 (0.58), 2102 | 2.0 (0.58), 4153 |
| Additional units transfused, median (IQR), n | 1.0 (0.0–3.0), 2060 | 1.0 (0.0–3.0), 2040 | 1.0 (0.0–3.0), 2017 | 1.0 (0.0–2.0), 2083 | 1.0 (0.0–3.0), 4100 |
| Platelet transfusions | |||||
| N | 868 | 790 | 811 | 847 | 1658 |
| Number of units transfused, mean (SD), n | 1.1 (0.39), 857 | 1.1 (0.58), 782 | 1.1 (0.41), 800 | 1.1 (0.56), 839 | 1.1 (0.49), 1639 |
| Additional units transfused, median (IQR), n | 2.0 (0.0–5.0), 854 | 2.0 (0.0–5.0), 779 | 3.0 (0.0–6.0), 798 | 2.0 (0.0–5.0), 835 | 2.0 (0.0–5.0), 1633 |
| Variable | Content | Follow-on | Total (N = 4439) | ||
|---|---|---|---|---|---|
| Standard (N = 2228) | Enhanced (N = 2211) | Standard (N = 2188) | Enhanced (N = 2251) | ||
| Age (years), median (IQR), n | 73.0 (64.0–80.0), 2227 | 72.0 (64.0–80.0), 2208 | 72.0 (64.0–80.0), 2187 | 72.0 (64.0–80.0), 2248 | 72.0 (64.0–80.0), 4435 |
| Gender (male), n (%) | 1306 (58.6) | 1335 (60.4) | 1301 (59.5) | 1340 (59.5) | 2641 (59.5) |
| Weight (kg), median (IQR), n | 71.0 (61.0–82.0), 1594 | 72.0 (62.5–83.9), 1565 | 72.0 (62.0–83.0), 1507 | 71.0 (62.0–82.8), 1652 | 71.5 (62.0–83.0), 3159 |
| Haematological diagnosis, n (%) | |||||
| Acute leukaemia | 469 (21.1) | 460 (20.8) | 435 (19.9) | 494 (21.9) | 929 (20.9) |
| Chronic leukaemia/lymphoma and myeloma | 751 (33.7) | 752 (34.0) | 754 (34.5) | 749 (33.3) | 1503 (33.9) |
| MDS and aplastic anaemia | 1038 (46.6) | 975 (44.1) | 1006 (46.0) | 1007 (44.7) | 2013 (45.3) |
| Additional treatment for haematological diagnosis, n (%) | 723 (32.5) | 615 (27.8) | 617 (28.2) | 721 (32.0) | 1338 (30.1) |
| Stem cell transplant | 128 (5.7) | 126 (5.7) | 94 (4.3) | 160 (7.1) | 254 (5.7) |
| Intensive chemotherapy | 574 (79.4) | 461 (75.0) | 494 (80.1) | 541 (75.0) | 1035 (77.4) |
| Participating in clinical study | 191 (8.6) | 166 (7.5) | 195 (8.9) | 162 (7.2) | 357 (8.0) |
| Receive a RBC transfusion, n (%) | 2104 (94.4) | 2064 (93.4) | 2060 (94.1) | 2108 (93.6) | 4168 (93.9) |
| Receive a platelet transfusion, n (%) | 868 (39.0) | 790 (35.7) | 811 (37.1) | 847 (37.6) | 1658 (37.4) |
| Transfusion type, n (%) | |||||
| RBCs and platelets | 744 (33.4) | 643 (29.1) | 683 (31.2) | 704 (31.3) | 1387 (31.2) |
| RBCs only | 1360 (61.0) | 1421 (64.3) | 1377 (62.9) | 1404 (62.4) | 2781 (62.6) |
| Platelets only | 124 (5.6) | 147 (6.6) | 128 (5.9) | 143 (6.4) | 271 (6.1) |
| RBC transfusions | |||||
| N | 2104 | 2064 | 2060 | 2108 | 4168 |
| Inpatient, n (%) | 697 (33.1) | 656 (31.8) | 698 (33.9) | 655 (31.1) | 1353 (32.5) |
| Symptoms | |||||
| Symptomatic anaemia, n (%) | 1079 (51.3) | 962 (46.6) | 1064 (51.7) | 977 (46.3) | 2041 (49.0) |
| Mild | 434 (40.2) | 395 (41.1) | 409 (38.4) | 420 (43.0) | 829 (40.6) |
| Moderate | 515 (47.7) | 443 (46.0) | 527 (49.5) | 431 (44.1) | 958 (46.9) |
| Severe | 87 (8.1) | 90 (9.4) | 99 (9.3) | 78 (8.0) | 177 (8.7) |
| Unspecified | 43 (4.0) | 34 (3.5) | 29 (2.8) | 48 (4.9) | 77 (3.8) |
| Haemoglobin level less than local threshold, n (%) | 581 (27.6) | 563 (27.3) | 557 (27.0) | 587 (27.8) | 1144 (27.4) |
| Chronic transfusion programme, n (%) | 532 (25.3) | 516 (25.0) | 539 (26.2) | 509 (24.1) | 1048 (25.1) |
| Cannot determine, n (%) | 41 (1.9) | 74 (3.6) | 42 (2.0) | 73 (3.5) | 115 (2.8) |
| Clinical indication, n (%) | |||||
| Acute blood loss | 38 (1.8) | 47 (2.3) | 43 (2.1) | 42 (2.0) | 85 (2.0) |
| Medical anaemia | 860 (38.6) | 706 (31.9) | 731 (33.4) | 835 (37.1) | 1566 (35.3) |
| Medical anaemia in patients with cardiovascular disease | 63 (3.0) | 58 (2.8) | 62 (3.0) | 59 (2.8) | 121 (2.9) |
| Medical anaemia with sepsis/CNS complications | 112 (5.3) | 103 (5.0) | 118 (5.7) | 97 (4.6) | 215 (5.2) |
| Medical anaemia when receiving radiotherapy | 8 (0.4) | 6 (0.3) | 7 (0.3) | 7 (0.3) | 14 (0.3) |
| Chronic anaemia | 1376 (65.4) | 1354 (65.6) | 1414 (68.6) | 1316 (62.4) | 2730 (65.5) |
| Other | 20 (1.0) | 34 (1.6) | 26 (1.3) | 28 (1.3) | 54 (1.3) |
| Number of units transfused, mean (SD), n | 1.9 (0.56), 2098 | 2.0 (0.59), 2055 | 1.9 (0.58), 2051 | 2.0 (0.58), 2102 | 2.0 (0.58), 4153 |
| Pre-transfusion haemoglobin count performed at an appropriate time?, n (%) | 1961 (93.2) | 1936 (93.8) | 1905 (92.5) | 1992 (94.5) | 3897 (93.5) |
| Pre-transfusion haemoglobin count, mean (SD), n | 78.9 (10.50), 1958 | 79.1 (11.26), 1930 | 79.3 (10.84), 1904 | 78.7 (10.92), 1984 | 79.0 (10.88), 3888 |
| Haemoglobin measured after each unit transfused, n (%) | 63 (3.6) | 56 (3.3) | 70 (4.2) | 49 (2.8) | 119 (3.5) |
| Post-transfusion haemoglobin count performed at an appropriate time?, n (%) | 674 (32.0) | 648 (31.4) | 679 (33.0) | 643 (30.5) | 1322 (31.7) |
| Additional units transfused, median (IQR), n | 1.0 (0.0–3.0), 2060 | 1.0 (0.0–3.0), 2040 | 1.0 (0.0–3.0), 2017 | 1.0 (0.0–2.0), 2083 | 1.0 (0.0–3.0), 4100 |
| Platelet transfusions | |||||
| N | 868 | 790 | 811 | 847 | 1658 |
| Inpatient, n (%) | 497 (57.3) | 472 (59.7) | 475 (58.6) | 494 (58.3) | 969 (58.4) |
| Reason for platelet transfusion, n (%) | |||||
| Prophylactic to prevent bleeding and not having a procedure modified WHO bleeding grade 0 or 1 | 677 (78.0) | 611 (77.3) | 629 (77.6) | 659 (77.8) | 1288 (77.7) |
| Pre-procedure modified WHO bleeding grade 0 or 1 as defined above | 74 (8.5) | 72 (9.1) | 75 (9.2) | 71 (8.4) | 146 (8.8) |
| Clinical indication, n (%) | |||||
| Prophylactic | 426 (49.1) | 422 (53.4) | 398 (49.1) | 450 (53.1) | 848 (51.1) |
| Prophylactic in the presence of currently existing risk factors for bleeding | 394 (45.4) | 315 (39.9) | 381 (47.0) | 328 (38.7) | 709 (42.8) |
| Pre-procedure | 79 (9.1) | 75 (9.5) | 79 (9.7) | 75 (8.9) | 154 (9.3) |
| Therapeutic | 89 (10.3) | 65 (8.2) | 73 (9.0) | 81 (9.6) | 154 (9.3) |
| Number of units transfused, mean (SD), n | 1.1 (0.39), 857 | 1.1 (0.58), 782 | 1.1 (0.41), 800 | 1.1 (0.56), 839 | 1.1 (0.49), 1639 |
| Platelets human leucocyte antigen matched, n (%) | 66 (7.6) | 51 (6.5) | 75 (9.2) | 42 (5.0) | 117 (7.1) |
| Pre-transfusion platelet count performed at an appropriate time?, n (%) | 819 (94.4) | 737 (93.3) | 762 (94.0) | 794 (93.7) | 1556 (93.8) |
| Pre-transfusion platelet count, median (IQR), n | 11.0 (8.0–18.0), 804 | 11.0 (8.0–18.0), 698 | 12.0 (8.0–18.0), 745 | 11.0 (8.0–18.0), 757 | 11.0 (8.0–18.0), 1502 |
| Platelet count above threshold stated in local guidelines, n (%) | 161 (19.7) | 187 (25.4) | 184 (24.1) | 164 (20.7) | 348 (22.4) |
| Platelet count measured after each unit transfused, n (%) | 29 (30.9) | 17 (19.8) | 28 (28.3) | 18 (22.2) | 46 (25.6) |
| Post-transfusion platelet count performed at an appropriate time?, n (%) | 531 (61.2) | 462 (58.5) | 488 (60.2) | 505 (59.6) | 993 (59.9) |
| Additional units transfused, median (IQR), n | 2.0 (0.0–5.0), 854 | 2.0 (0.0–5.0), 779 | 3.0 (0.0–6.0), 798 | 2.0 (0.0–5.0), 835 | 2.0 (0.0–5.0), 1633 |
| Variable | Content | Follow-on | Total (N = 3859) | ||
|---|---|---|---|---|---|
| Standard (N = 1926) | Enhanced (N = 1933) | Standard (N = 1779) | Enhanced (N = 2080) | ||
| Age (years), median (IQR), n | 73.0 (64.0–81.0), 1926 | 73. (63.0–81.0), 1933 | 73.0 (63.0–81.0), 1779 | 73.0 (63.0–81.0), 2080 | 73.0 (63.0–81.0), 3859 |
| Gender (male), n (%) | 1130 (58.7) | 1166 (60.3) | 719 (40.4) | 844 (40.6) | 2296 (59.5) |
| Weight (kg), median (IQR), n | 72.0 (63.1–82.5), 1447 | 72.4 (63.0–83.0), 1267 | 72.2 (62.7–83.0), 1222 | 72.0 (63.4–82.0), 1492 | 72.0 (63.0–83.0), 2714 |
| Haematological diagnosis, n (%) | |||||
| Acute leukaemia | 430 (22.3) | 381 (19.7) | 348 (19.6) | 463 (22.3) | 811 (21.0) |
| Chronic leukaemia/lymphoma and myeloma | 625 (32.5) | 603 (31.2) | 591 (33.2) | 637 (30.6) | 1228 (31.8) |
| MDS and aplastic anaemia | 874 (45.4) | 894 (46.2) | 827 (46.5) | 941 (45.2) | 1768 (45.8) |
| Additional treatment for haematological diagnosis, n (%) | 584 (30.3) | 539 (27.9) | 508 (28.6) | 615 (29.6) | 1123 (29.1) |
| Stem cell transplant | 93 (4.8) | 94 (4.9) | 93 (5.2) | 94 (4.5) | 187 (4.8) |
| Intensive chemotherapy | 449 (76.9) | 407 (75.5) | 379 (74.6) | 477 (77.6) | 856 (76.2) |
| Participating in clinical study | 170 (8.8) | 150 (7.8) | 181 (10.2) | 139 (6.7) | 320 (8.3) |
| Receive a RBC transfusion, n (%) | 1815 (94.2) | 1832 (94.8) | 1674 (94.1) | 1973 (94.9) | 3647 (94.5) |
| Receive a platelet transfusion, n (%) | 717 (37.2) | 702 (36.3) | 621 (34.9) | 798 (38.4) | 1419 (36.8) |
| Transfusion type, n (%) | |||||
| RBCs and platelets | 606 (31.5) | 601 (31.1) | 516 (29.0) | 691 (33.2) | 1207 (31.3) |
| RBCs only | 1209 (62.8) | 1231 (63.7) | 1158 (65.1) | 1282 (61.6) | 2440 (63.2) |
| Platelets only | 111 (5.8) | 101 (5.2) | 105 (5.9) | 107 (5.1) | 212 (5.5) |
| RBC transfusions | |||||
| N | 1815 | 1832 | 1674 | 1973 | 3647 |
| Inpatient, n (%) | 564 (31.1) | 547 (29.9) | 528 (31.5) | 583 (29.5) | 1111 (30.5) |
| Symptoms | |||||
| Symptomatic anaemia, n (%) | 821 (45.2) | 830 (45.3) | 789 (47.1) | 862 (43.7) | 1651 (45.3) |
| Mild | 332 (40.4) | 346 (41.7) | 337 (42.7) | 341 (39.6) | 678 (41.1) |
| Moderate | 375 (45.7) | 359 (43.3) | 341 (43.2) | 393 (45.6) | 734 (44.5) |
| Severe | 73 (8.9) | 68 (8.2) | 67 (8.5) | 74 (8.6) | 141 (8.5) |
| Unspecified | 41 (5.0) | 57 (6.8) | 44 (5.6) | 54 (6.2) | 98 (5.9) |
| Haemoglobin level less than local threshold, n (%) | 505 (27.8) | 487 (26.6) | 467 (27.9) | 525 (26.6) | 992 (27.2) |
| Chronic transfusion programme, n (%) | 607 (33.4) | 578 (31.6) | 535 (32.0) | 650 (32.9) | 1185 (32.5) |
| Cannot determine, n (%) | 54 (3.0) | 80 (4.4) | 52 (3.1) | 82 (4.2) | 134 (3.7) |
| Clinical indication, n (%) | |||||
| Acute blood loss | 29 (1.6) | 40 (2.2) | 30 (1.8) | 39 (2.0) | 69 (1.9) |
| Medical anaemia | 776 (40.3) | 733 (37.9) | 662 (37.2) | 847 (40.7) | 1509 (39.1) |
| Medical anaemia in patients with cardiovascular disease | 76 (4.2) | 59 (3.2) | 69 (4.1) | 66 (3.3) | 135 (3.7) |
| Medical anaemia with sepsis/CNS complications | 100 (5.5) | 103 (5.6) | 109 (6.5) | 94 (4.8) | 203 (5.6) |
| Medical anaemia when receiving radiotherapy | 24 (1.3) | 5 (0.3) | 5 (0.3) | 24 (1.2) | 29 (0.8) |
| Chronic anaemia | 1172 (64.6) | 1126 (61.5) | 1083 (64.7) | 1215 (61.6) | 2298 (63.0) |
| Other | 51 (2.8) | 66 (3.6) | 74 (4.4) | 43 (2.2) | 117 (3.2) |
| Number of units transfused, mean (SD), n | 1.8 (0.65), 1813 | 1.8 (0.60), 1829 | 1.8 (0.62), 1671 | 1.8 (0.63), 1971 | 1.8 (0.62), 3642 |
| Pre-transfusion haemoglobin count performed at an appropriate time?, n (%) | 1713 (94.4) | 1706 (93.1) | 1571 (93.8) | 1848 (93.7) | 3419 (93.7) |
| Pre-transfusion haemoglobin count, mean (SD), n | 77.2 (9.88), 1713 | 78.0 (10.11), 1705 | 77.3 (9.84), 1571 | 77.8 (10.14), 1847 | 77.6 (10.01), 3418 |
| Haemoglobin measured after each unit transfused, n (%) | 63 (4.9) | 39 (3.1) | 50 (4.3) | 52 (3.7) | 102 (4.0) |
| Post-transfusion haemoglobin count performed at an appropriate time?, n (%) | 600 (33.1) | 568 (31.0) | 557 (33.3) | 611 (31.0) | 1168 (32.0) |
| Additional units transfused, median (IQR), n | 1.0 (0.0–3.0), 1772 | 1.0 (0.0–3.0), 1770 | 1.0 (0.0–2.0), 1635 | 1.0 (0.0–3.0), 1907 | 1.0 (0.0–3.0), 3542 |
| Platelet transfusions | |||||
| N | 717 | 702 | 621 | 798 | 1419 |
| Inpatient, n (%) | 399 (55.6) | 378 (53.8) | 352 (56.7) | 425 (53.3) | 777 (54.8) |
| Reason for platelet transfusion, n (%) | |||||
| Prophylactic to prevent bleeding and not having a procedure modified WHO bleeding grade 0 or 1 | 562 (78.4) | 557 (79.3) | 506 (81.5) | 613 (76.8) | 1119 (78.9) |
| Pre-procedure modified WHO bleeding grade 0 or 1 as defined above | 68 (9.5) | 60 (8.5) | 49 (7.9) | 79 (9.9) | 128 (9.0) |
| Clinical indication, n (%) | |||||
| Prophylactic | 329 (45.9) | 328 (46.7) | 282 (45.4) | 375 (47.0) | 657 (46.3) |
| Prophylactic in the presence of currently existing risk factors for bleeding | 289 (40.3) | 295 (42.0) | 270 (43.5) | 314 (39.3) | 584 (41.2) |
| Pre-procedure | 70 (9.8) | 65 (9.3) | 53 (8.5) | 82 (10.3) | 135 (9.5) |
| Therapeutic | 59 (8.2) | 55 (7.8) | 50 (8.1) | 64 (8.0) | 114 (8.0) |
| Number of units transfused, mean (SD), n | 1.1 (0.37), 704 | 1.1 (0.60), 690 | 1.1 (0.37), 614 | 1.1 (0.58), 780 | 1.1 (0.50), 1394 |
| Platelets human leucocyte antigen matched, n (%) | 37 (5.2) | 61 (8.7) | 50 (8.1) | 48 (6.0) | 98 (6.9) |
| Pre-transfusion platelet count performed at an appropriate time?, n (%) | 692 (96.5) | 675 (96.2) | 601 (96.8) | 766 (96.0) | 1367 (96.3) |
| Pre-transfusion platelet count, median (IQR), n | 11.0 (7.0–17.0), 688 | 11.0 (8.0–18.0), 667 | 11.0 (7.0–18.0), 597 | 11.0 (7.0–18.0), 758 | 11.0 (7.0–18.0), 1355 |
| Platelet count above threshold stated in local guidelines, n (%) | 154 (22.3) | 173 (25.6) | 166 (27.6) | 161 (21.0) | 327 (23.9) |
| Platelet count measured after each unit transfused, n (%) | 30 (45.5) | 11 (21.6) | 20 (39.2) | 21 (31.8) | 41 (35.0) |
| Post-transfusion platelet count performed at an appropriate time?, n (%) | 448 (62.5) | 414 (59.0) | 392 (63.1) | 470 (58.9) | 862 (60.7) |
| Additional units transfused, median (IQR), n | 2.0 (1.0–5.0), 700 | 2.0 (1.0–5.0), 673 | 2.0 (1.0–5.0), 607 | 2.0 (1.0–5.0), 766 | 2.0 (1.0–5.0), 1373 |
| Variable | Content | Follow-on | Total (N = 4439) | ||
|---|---|---|---|---|---|
| Standard (N = 2228) | Enhanced (N = 2211) | Standard (N = 2188) | Enhanced (N = 2251) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 1355 (60.8) | 1396 (63.1) | 1393 (63.7) | 1358 (60.3) | 2751 (62.0) |
| Outside guidelines | 617 (27.7) | 524 (23.7) | 544 (24.9) | 597 (26.5) | 1141 (25.7) |
| Unclassified | 256 (11.5) | 291 (13.2) | 251 (11.5) | 296 (13.1) | 547 (12.3) |
| Supportive outcomes,a n (%) | |||||
| Standard 1 | |||||
| Meets standard | 1961 (93.4) | 1936 (94.0) | 1905 (92.7) | 1992 (94.6) | 3897 (93.7) |
| Does not meet standard | 139 (6.6) | 124 (6.0) | 149 (7.3) | 114 (5.4) | 263 (6.3) |
| Standard 2 | |||||
| Meets standard | 148 (25.8) | 140 (25.3) | 142 (24.9) | 146 (26.2) | 288 (25.6) |
| Does not meet standard | 415 (72.4) | 405 (73.1) | 422 (74.0) | 398 (71.5) | 820 (72.8) |
| Insufficient information | 10 (1.7) | 9 (1.6) | 6 (1.1) | 13 (2.3) | 19 (1.7) |
| Standard 3 | |||||
| Meets standard | 9 (40.9) | 11 (68.8) | 13 (54.2) | 7 (50.0) | 20 (52.6) |
| Does not meet standard | 13 (59.1) | 5 (31.3) | 11 (45.8) | 7 (50.0) | 18 (47.4) |
| Standard 6 | |||||
| Meets standard | 120 (53.6) | 99 (49.3) | 102 (52.8) | 117 (50.4) | 219 (51.5) |
| Does not meet standard | 95 (42.4) | 82 (40.8) | 82 (42.5) | 95 (40.9) | 177 (41.6) |
| Insufficient information | 9 (4.0) | 20 (10.0) | 9 (4.7) | 20 (8.6) | 29 (6.8) |
| Standard 7 | |||||
| Meets standard | 405 (59.8) | 391 (64.0) | 373 (59.3) | 423 (64.2) | 796 (61.8) |
| Does not meet standard | 272 (40.2) | 220 (36.0) | 256 (40.7) | 236 (35.8) | 492 (38.2) |
| RBC transfusions | (n = 2104) | (n = 2064) | (n = 2060) | (n = 2108) | (n = 4168) |
| Secondary outcome, median (IQR), n | |||||
| Volume of RBCs transfused | 2.0 (2.0–2.0), 2098 | 2.0 (2.0–2.0), 2055 | 2.0 (2.0–2.0), 2051 | 2.0 (2.0–2.0), 2102 | 2.0 (2.0–2.0), 4153 |
| Supportive outcomes, n (%) | |||||
| RBC component of primary outcome | |||||
| Acceptable | 1627 (77.3) | 1605 (77.8) | 1651 (80.1) | 1581 (75.0) | 3232 (77.5) |
| Outside guidelines | 262 (12.5) | 213 (10.3) | 198 (9.6) | 277 (13.1) | 475 (11.4) |
| Unclassified | 215 (10.2) | 246 (11.9) | 211 (10.2) | 250 (11.9) | 461 (11.1) |
| Intermediate outcomes, n (%) | |||||
| Haemoglobin measured after each unit transfused | 63 (3.6) | 56 (3.3) | 70 (4.2) | 49 (2.8) | 119 (3.5) |
| Platelet transfusions | (n = 883) | (n = 808) | (n = 826) | (n = 865) | (n = 1691) |
| Secondary outcome, median (IQR), n | |||||
| Volume of platelets transfused | 1.0 (1.0–1.0), 872 | 1.0 (1.0–1.0), 800 | 1.0 (1.0–1.0), 815 | 1.0 (1.0–1.0), 857 | 1.0 (1.0–1.0), 1672 |
| Supportive outcomes, n (%) | |||||
| Platelet component of primary outcome | |||||
| Acceptable | 278 (31.5) | 256 (31.7) | 257 (31.1) | 277 (32.0) | 534 (31.6) |
| Outside guidelines | 406 (46.0) | 346 (42.8) | 384 (46.5) | 368 (42.5) | 752 (44.5) |
| Unclassified | 199 (22.5) | 206 (25.5) | 185 (22.4) | 220 (25.4) | 405 (24.0) |
| Intermediate outcomes, n (%) | |||||
| Platelet count measured after each unit transfused | 29 (30.2) | 18 (20.7) | 29 (28.4) | 18 (22.2) | 47 (25.7) |
| Variable | Content | Follow-on | Total (N = 3859) | ||
|---|---|---|---|---|---|
| Standard (N = 1926) | Enhanced (N = 1933) | Standard (N = 1779) | Enhanced (N = 2080) | ||
| Primary outcome, n (%) | |||||
| Acceptable | 1308 (67.9) | 1226 (63.4) | 1196 (67.2) | 1338 (64.3) | 2534 (65.7) |
| Outside guidelines | 457 (23.7) | 507 (26.2) | 433 (24.3) | 531 (25.5) | 964 (25.0) |
| Unclassified | 161 (8.4) | 200 (10.3) | 150 (8.4) | 211 (10.1) | 361 (9.4) |
| Supportive outcomes, n (%) | |||||
| Standard 1 | |||||
| Meets standard | 1713 (94.5) | 1706 (93.2) | 1571 (94.0) | 1848 (93.7) | 3419 (93.9) |
| Does not meet standard | 100 (5.5) | 124 (6.8) | 100 (6.0) | 124 (6.3) | 224 (6.1) |
| Standard 2 | |||||
| Meets standard | 138 (30.0) | 102 (23.4) | 118 (28.5) | 122 (25.3) | 240 (26.8) |
| Does not meet standard | 318 (69.1) | 325 (74.5) | 291 (70.3) | 352 (73.0) | 643 (71.8) |
| Insufficient information | 4 (0.9) | 9 (2.1) | 5 (1.2) | 8 (1.7) | 13 (1.5) |
| Standard 3 | |||||
| Meets standard | 8 (50.0) | 15 (83.3) | 8 (53.3) | 15 (78.9) | 23 (67.6) |
| Does not meet standard | 6 (37.5) | 1 (5.6) | 5 (33.3) | 2 (10.5) | 7 (20.6) |
| Insufficient information | 2 (12.5) | 2 (11.1) | 2 (13.3) | 2 (10.5) | 4 (11.8) |
| Standard 6 | |||||
| Meets standard | 107 (59.1) | 105 (53.8) | 85 (57.0) | 127 (55.9) | 212 (56.4) |
| Does not meet standard | 72 (39.8) | 87 (44.6) | 62 (41.6) | 97 (42.7) | 159 (42.3) |
| Insufficient information | 2 (1.1) | 3 (1.5) | 2 (1.3) | 3 (1.3) | 5 (1.3) |
| Standard 7 | |||||
| Meets standard | 333 (59.3) | 381 (68.4) | 311 (61.5) | 403 (65.7) | 714 (63.8) |
| Does not meet standard | 229 (40.7) | 176 (31.6) | 195 (38.5) | 210 (34.3) | 405 (36.2) |
| RBC transfusions | (n = 1815) | (n = 1832) | (n = 1674) | (n = 1973) | (n = 3647) |
| Secondary outcome, median (IQR), n | |||||
| Volume of RBCs transfused (units) | 2.0 (1.0–2.0), 1813 | 2.0 (1.0–2.0), 1829 | 2.0 (1.0–2.0), 1671 | 2.0 (1.0–2.0), 1971 | 2.0 (1.0–2.0), 3642 |
| Supportive outcomes,a n (%) | |||||
| RBC component of primary outcome | |||||
| Acceptable | 1497 (82.5) | 1438 (78.5) | 1368 (81.7) | 1567 (79.4) | 2935 (80.5) |
| Outside guidelines | 173 (9.5) | 209 (11.4) | 169 (10.1) | 213 (10.8) | 382 (10.5) |
| Unclassified | 145 (8.0) | 185 (10.1) | 137 (8.2) | 193 (9.8) | 330 (9.0) |
| Intermediate outcomes, n (%) | |||||
| Haemoglobin measured after each unit transfused | 63 (4.9) | 39 (3.1) | 50 (4.3) | 52 (3.7) | 102 (4.0) |
| Platelet transfusions | |||||
| N | 729 | 717 | 633 | 813 | 1446 |
| Secondary outcome, median (IQR), n | |||||
| Volume of platelets transfused (units) | 1.0 (1.0–1.0), 716 | 1.0 (1.0–1.0), 705 | 1.0 (1.0–1.0), 626 | 1.0 (1.0–1.0), 795 | 1.0 (1.0–1.0), 1421 |
| Supportive outcomes, n (%) | |||||
| Platelet component of primary outcome | |||||
| Acceptable | 279 (38.3) | 241 (33.6) | 227 (35.9) | 293 (36.0) | 520 (36.0) |
| Outside guidelines | 315 (43.2) | 337 (47.0) | 299 (47.2) | 353 (43.4) | 652 (45.1) |
| Unclassified | 135 (18.5) | 139 (19.3) | 107 (16.9) | 167 (20.6) | 174 (19.0) |
| Intermediate outcomes, n (%) | |||||
| Platelet count measured after each unit transfused | 30 (45.5) | 11 (21.6) | 20 (39.2) | 21 (31.8) | 41 (35.0) |
| Analysis | Unadjusted proportion acceptable | Estimated adjusted risk difference (95% CI) | Estimated adjusted odds ratio (97.5% CI) | Estimated adjusted odds ratio (95% CI) | p-value | n | |
|---|---|---|---|---|---|---|---|
| Standard | Enhanced | ||||||
| Primary analysis (multiple imputation, 100 imputations, full imputation model) | |||||||
| Content | 0.744 | 0.714 | –0.04 (–0.09 to 0.02) | 0.82 (0.59 to 1.15) | 0.82 (0.61 to 1.10) | 0.193 | 3859 |
| Follow-on | 0.739 | 0.721 | –0.01 (–0.06 to 0.05) | 0.96 (0.67 to 1.38) | 0.96 (0.70 to 1.32) | 0.811 | 3859 |
| Interaction | 0.737 | 0.707 | 0.02 (–0.10 to 0.13) | 1.15 (0.56 to 2.34) | 1.15 (0.61 to 2.14) | 0.668 | 3859 |
| Sensitivity analyses | |||||||
| Complete-case analysis | |||||||
| Content | 0.741 | 0.707 | –0.04 (–0.09 to 0.02) | 0.82 (0.59 to 1.15) | 0.82 (0.61 to 1.10) | 0.193 | 3498 |
| Follow-on | 0.734 | 0.716 | –0.01 (–0.06 to 0.05) | 0.96 (0.67 to 1.38) | 0.96 (0.70 to 1.32) | 0.811 | 3498 |
| Interaction | 0.734 | 0.699 | 0.02 (–0.10 to 0.13) | 1.15 (0.56 to 2.34) | 1.15 (0.61 to 2.14) | 0.668 | 3498 |
| Analysis | Unadjusted proportion acceptable | Estimated adjusted risk difference (95% CI) | p-value | n | |
|---|---|---|---|---|---|
| Standard | Enhanced | ||||
| RBC component of the primary outcomea | |||||
| Content | 0.889 | 0.869 | 0.73 (0.45 to 1.18) | 0.204 | 3647 |
| Follow-on | 0.887 | 0.873 | 0.92 (0.52 to 1.61) | 0.767 | 3647 |
| Interaction | 0.886 | 0.862 | 1.30 (0.46 to 3.69) | 0.623 | 3647 |
| Platelet component of the primary outcomea,b | |||||
| Content | 0.476 | 0.422 | 0.77 (0.54 to 1.11) | 0.167 | 1247 |
| Follow-on | 0.437 | 0.459 | 1.11 (0.78 to 1.60) | 0.558 | 1247 |
| Interaction | 0.445 | 0.459 | 1.90 (0.92 to 3.91) | 0.083 | 1247 |
| NCA standard 1b,c,d | |||||
| Content | 0.944 | 0.932 | 0.71 (0.43 to 1.19) | 0.194 | 3643 |
| Follow-on | 0.940 | 0.937 | 0.97 (0.58 to 1.61) | 0.897 | 3643 |
| Interaction | 0.942 | 0.930 | 0.97 (0.35 to 2.70) | 0.957 | 3643 |
| NCA standard 2a,b | |||||
| Content | 0.304 | 0.237 | 0.66 (0.44 to 0.98) | 0.041 | 896 |
| Follow-on | 0.287 | 0.257 | 1.01 (0.68 to 1.52) | 0.947 | 896 |
| Interaction | 0.279 | 0.250 | 1.89 (0.84 to 4.25) | 0.121 | 896 |
| NCA standard 6a,b | |||||
| Content | 0.598 | 0.548 | 0.71 (0.40 to 1.25) | 0.235 | 376 |
| Follow-on | 0.579 | 0.567 | 1.07 (0.61 to 1.90) | 0.809 | 376 |
| Interaction | 0.560 | 0.598 | 3.59 (1.25 to 11.31) | 0.029 | 376 |
| NCA standard 7b,c | |||||
| Content | 0.593 | 0.684 | 1.37 (0.89 to 2.10) | 0.146 | 1119 |
| Follow-on | 0.615 | 0.657 | 0.94 (0.62 to 1.45) | 0.792 | 1119 |
| Interaction | 0.612 | 0.706 | 1.29 (0.55 to 3.04) | 0.554 | 1119 |
| Variable | Content | Follow-on | Total (N = 134) | ||
|---|---|---|---|---|---|
| Standard (N = 68) | Enhanced (N = 66) | Standard (N = 67) | Enhanced (N = 67) | ||
| Total number of incidents, median (IQR) (range) | |||||
| Baseline | 12 (6.5–25) (0–189) | 14 (7–21) (0–71) | 15 (9–25) (0–56) | 12 (6–22) (0–189) | 13 (7–24) (0–189) |
| Follow-up | 15.5 (7–25) (0–175) | 15 (8–26) (0–74) | 16 (8–26) (0–74) | 13 (6–25) (0–175) | 15 (8–25) (0–175) |
| July–September 2015 (baseline) | 3 (2–6) (0–47) | 4.5 (2–7) (0–24) | 4 (3–7) (0–16) | 3 (1–7) (0–47) | 4 (2–7) (0–47) |
| October–December 2015 (baseline) | 3 (1–6) (0–71) | 3 (1–6) (0–18) | 3 (1–6) (0–32) | 3 (1–6) (0–71) | 3 (1–6) (0–71) |
| January–March 2016 (baseline) | 3.5 (2–6) (0–39) | 3 (1–6) (0–14) | 3 (2–6) (0–13) | 3 (1–6) (0–39) | 3 (2–6) (0–39) |
| April–June 2016 (baseline) | 3 (1–6) (0–32) | 3 (1–5) (0–23) | 3 (2–6) (0–16) | 2 (1–6) (0–32) | 3 (1–6) (0–32) |
| July–September 2016 (follow-up) | 4 (2–6) (0–33) | 3.5 (2–7) (0–18) | 4 (2–6) (0–17) | 4 (2–7) (0–33) | 4 (2–7) (0–33) |
| October–December 2016 (follow-up) | 3 (1–6.5) (0–55) | 3.5 (2–8) (0–26) | 4 (2–8) (0–26) | 2 (1–7) (0–55) | 3 (1–7) (0–55) |
| January–March 2017 (follow-up) | 4 (1.5–7) (0–47) | 3 (2–6) (0–22) | 4 (2–7) (0–18) | 3 (1–6) (0–47) | 4 (2–6) (0–47) |
| April–June 2017 (follow-up) | 3 (1–6) (0–43) | 3 (2–7) (0–18) | 3 (2–6) (0–18) | 3 (1–7) (0–43) | 3 (1–6) (0–43) |
| Number of relevant errors, median (IQR) (range) | |||||
| Baseline | 1 (0–1.5) (0–11) | 1 (0–1) (0–11) | 1 (0–2) (0–11) | 0 (0–1) (0–11) | 1 (0–1) (0–11) |
| Follow-up | 0 (0–1) (0–8) | 0 (0–1) (0–21) | 0 (0–1) (0–4) | 0 (0–1) (0–21) | 0 (0–1) (0–21) |
| July–September 2015 (baseline) | 0 (0–0) (0–2) | 0 (0–1) (0–4) | 0 (0–1) (0–4) | 0 (0–0) (0–2) | 0 (0–0) (0–4) |
| October–December 2015 (baseline) | 0 (0–0.5) (0–4) | 0 (0–0) (0–11) | 0 (0–0) (0–11) | 0 (0–0) (0–4) | 0 (0–0) (0–11) |
| January–March 2016 (baseline) | 0 (0–0) (0–4) | 0 (0–0) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–4) | 0 (0–0) (0–4) |
| April–June 2016 (baseline) | 0 (0–0) (0–3) | 0 (0–0) (0–4) | 0 (0–0) (0–4) | 0 (0–0) (0–4) | 0 (0–0) (0–4) |
| July–September 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) |
| October–December 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| January–March 2017 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–21) | 0 (0–0) (0–1) | 0 (0–0) (0–21) | 0 (0–0) (0–21) |
| April–June 2017 (follow-up) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) |
| Number of relevant near-miss for ‘right blood right patient’ incidents, median (IQR) (range) | |||||
| Baseline | 0 (0–1) (0–3) | 0 (0–0) (0–3) | 0 (0–1) (0–1) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| Follow-up | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| July–September 2015 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| October–December 2015 (baseline) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| January–March 2016 (baseline) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| April–June 2016 (baseline) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| July–September 2016 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| October–December 2016 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| January–March 2017 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–1) | 0 (0–0) (0–2) | 0 (0–0) (0–2) |
| April–June 2017 (follow-up) | 0 (0–0) (0–1) | 0 (0–0) (0–0) | 0 (0–0) (0–0) | 0 (0–0) (0–1) | 0 (0–0) (0–1) |
| Total number of unpredictable incidents, median (IQR) (range) | |||||
| Baseline | 1 (0–2.5) (0–11) | 1 (0–2) (0–15) | 1 (0–3) (0–7) | 1 (0–2) (0–15) | 1 (0–2) (0–15) |
| Follow-up | 1 (0–2.5) (0–9) | 1 (0–3) (0–16) | 1 (0–3) (0–16) | 1 (0–2) (0–9) | 1 (0–3) (0–16) |
| July–September 2015 (baseline) | 0 (0–1) (0–5) | 0 (0–1) (0–5) | 0 (0–1) (0–3) | 0 (0–0) (0–5) | 0 (0–1) (0–5) |
| October–December 2015 (baseline) | 0 (0–1) (0–4) | 0 (0–1) (0–2) | 0 (0–0) (0–4) | 0 (0–1) (0–2) | 0 (0–1) (0–4) |
| January–March 2016 (baseline) | 0 (0–0) (0–4) | 0 (0–1) (0–3) | 0 (0–1) (0–4) | 0 (0–0) (0–3) | 0 (0–1) (0–4) |
| April–June 2016 (baseline) | 0 (0–1) (0–3) | 0 (0–0) (0–6) | 0 (0–1) (0–3) | 0 (0–1) (0–6) | 0 (0–1) (0–6) |
| July–September 2016 (follow-up) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–0) (0–3) | 0 (0–1) (0–3) |
| October–December 2016 (follow-up) | 0 (0–0.5) (0–4) | 0 (0–1) (0–6) | 0 (0–0) (0–6) | 0 (0–1) (0–4) | 0 (0–1) (0–6) |
| January–March 2017 (follow-up) | 0 (0–0) (0–3) | 0 (0–1) (0–8) | 0 (0–1) (0–8) | 0 (0–0) (0–4) | 0 (0–1) (0–8) |
| April–June 2017 (follow-up) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–1) (0–3) | 0 (0–0) (0–3) | 0 (0–1) (0–3) |
| Total number of possibly preventable incidents, median (IQR) (range) | |||||
| Baseline | 0 (0–2) (0–48) | 0 (0–1) (0–6) | 1 (0–1) (0–7) | 0 (0–2) (0–48) | 0 (0–2) (0–48) |
| Follow-up | 0 (0–1.5) (0–13) | 0 (0–1) (0–8) | 0 (0–1) (0–6) | 0 (0–1) (0–13) | 0 (0–1) (0–13) |
| July–September 2015 (baseline) | 0 (0–0.5) (0–18) | 0 (0–0) (0–5) | 0 (0–1) (0–5) | 0 (0–0) (0–18) | 0 (0–0) (0–18) |
| October–December 2015 (baseline) | 0 (0–0) (0–28) | 0 (0–1) (0–4) | 0 (0–0) (0–6) | 0 (0–0) (0–28) | 0 (0–0) (0–28) |
| January–March 2016 (baseline) | 0 (0–0) (0–2) | 0 (0–0) (0–3) | 0 (0–0) (0–1) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| April–June 2016 (baseline) | 0 (0–0) (0–3) | 0 (0–0) (0–1) | 0 (0–0) (0–3) | 0 (0–0) (0–2) | 0 (0–0) (0–3) |
| July–September 2016 (follow-up) | 0 (0–0) (0–3) | 0 (0–0) (0–7) | 0 (0–0) (0–3) | 0 (0–0) (0–7) | 0 (0–0) (0–7) |
| October–December 2016 (follow-up) | 0 (0–0) (0–4) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–4) | 0 (0–0) (0–4) |
| January–March 2017 (follow-up) | 0 (0–0) (0–3) | 0 (0–1) (0–2) | 0 (0–1) (0–3) | 0 (0–0) (0–3) | 0 (0–0) (0–3) |
| April–June 2017 (follow-up) | 0 (0–0) (0–4) | 0 (0–0) (0–2) | 0 (0–0) (0–2) | 0 (0–0) (0–4) | 0 (0–0) (0–4) |
| Variable | Content | Follow-on | Total (N = 134) | ||
|---|---|---|---|---|---|
| Standard (N = 68) | Enhanced (N = 66) | Standard (N = 67) | Enhanced (N = 67) | ||
| Total volume of RBCs transfused, median (IQR) (range), n | |||||
| Baseline | 7724.5 (5298–11,183) (1024–30,449), 66 | 7662 (5243–11,838) (1764–38,434), 65 | 6747 (4730.5–11,247.5) (1727–38,434), 64 | 8532 (5316–11,838) (1024–30,449), 67 | 7662 (5243–11,514) (1024–38,434), 131 |
| Follow-up | 6793 (4843–11,481) (1216–29,892), 65 | 7560 (4677–11,483) (1263–29,014), 66 | 6480 (4479.5–11,514.5) (1263–29,892), 64 | 7769 (4843–10,977) (1216–29,014), 67 | 7107 (4677–11,483) (1216–29,892), 131 |
| July–September 2015 (baseline) | 2026.5 (1406.5–2782) (433–7655), 64 | 2037.5 (1345–2907) (712–10,604), 62 | 1896 (1278–2771) (712–10,604), 61 | 2137 (1418–2946) (433–7655), 65 | 2026.5 (1375–2878) (433–10,604), 126 |
| October–December 2015 (baseline) | 1994 (1370–2883) (687–7897), 63 | 2154 (1370–2917) (368–9865), 63 | 1889 (1188–2858) (368–9865), 61 | 2198 (1439–2917) (687–7897), 65 | 2025 (1370–2883) (368–9865), 126 |
| January–March 2016 (baseline) | 2060.5 (1269–2713.5) (648–7655), 64 | 2003.5 (1224.5–2999.5) (535–9841), 64 | 1826.5 (1143–2689) (648–9841), 62 | 2101.5 (1274–2929) (535–8020), 66 | 2030 (1247.5–2822.5) (535–9841), 128 |
| April–June 2016 (baseline) | 1987 (1293–2665) (275–7637), 65 | 1946.5 (1276.5–3023) (671–8124), 64 | 1937 (1246–3169) (671–8124), 63 | 2105 (1293–3001) (275–7242), 66 | 1965 (1288–3001) (275–8124), 129 |
| July–September 2016 (follow-up) | 1818.5 (1257.5–2979.5) (352–7912), 64 | 2088 (1176–3114) (618–7680), 63 | 1818.5 (1153–2984) (618–7912), 62 | 2088 (1329–2836) (352–7164), 65 | 2019 (1214–2984) (352–7912), 127 |
| October–December 2016 (follow-up) | 1791 (1222–2722) (441–7453), 65 | 1953 (1274–2926) (384–7618), 63 | 1850 (1097–2859) (384–7453), 62 | 1939 (1300–2722) (441–7618), 66 | 1927 (1231–2843) (384–7618), 128 |
| January–March 2017 (follow-up) | 1898 (1275–2908) (323–7145), 63 | 1930 (1145–2940) (659–7683), 63 | 1811.5 (1094–2913) (627–7145), 62 | 1955.5 (1300.5–3025) (323–7683), 64 | 1918.5 (1197–2913) (323–7683), 126 |
| April–June 2017 (follow-up) | 1733 (1240.5–2861.5) (268–7382), 64 | 1794 (1152–2995.5) (473–7786), 64 | 1558 (1141–2861.5) (473–7382), 64 | 1886.5 (1273–3071) (268–7786), 64 | 1769.5 (1194.5–2869.5) (268–7786), 128 |
| Total gross volume of RBCs issued, median (IQR) (range), n | |||||
| Baseline | 8489 (5456–11,667) (2823–31,009), 7 | 8483.5 (5599–11,925) (3135–41,792), 66 | 8203.5 (5483–11,667) (2968–41,792), 66 | 8760 (5535–11,925) (2823–32,230), 67 | 8489 (5520–11,784) (2823–41,792), 133 |
| Follow-up | 7996 (4958–11,480) (2900–30,850), 67 | 8213 (5096–11,800) (2801–43,714), 66 | 7816 (4958–11,678) (2801–43,714), 66 | 8129 (5108–11,746) (2900–32,254), 67 | 8050 (5096–11,678) (2801–43,714), 133 |
| July–September 2015 (baseline) | 2220 (1432–2933) (704–7811), 67 | 2139 (1416–2987) (738–11,013), 66 | 2099.5 (1416–2956) (738–11,013), 66 | 2202 (1455–3025) (704–8126), 67 | 2144 (1423–2965) (704–11,013), 133 |
| October–December 2015 (baseline) | 2064 (1348–2891) (713–8029), 67 | 2167.5 (1433–3026) (782–10,292), 66 | 2038 (1315–2970) (780–10,292), 66 | 2225 (1433–2930) (713–8029), 67 | 2118 (1425–2948) (713–10,292), 133 |
| January–March 2016 (baseline) | 2159 (1353–2838) (681–7782), 67 | 2038 (1428–2889) (782–10,187), 66 | 2080 (1381–2835) (681–10,187), 66 | 2159 (1392–3060) (694–8283), 67 | 2115 (1392–2838) (681–10,187), 133 |
| April–June 2016 (baseline) | 2122 (1316–2850) (712–7812), 67 | 2119 (1435–3050) (774–10,300), 66 | 2039.5 (1316–2937) (732–10,300), 66 | 2220 (1393–3021) (712–8221), 67 | 2122 (1351–2945) (712–10,300), 133 |
| July–September 2016 (follow-up) | 1998 (1282–2916) (745–8099), 67 | 2115.5 (1340–3003) (647–10,927), 66 | 1926 (1282–3003) (647–10,927), 66 | 2192 (1296–2916) (745–8053), 67 | 2104 (1296–2954) (647–10,927), 133 |
| October–December 2016 (follow-up) | 2013 (1288–2870) (742–7646), 67 | 2041.5 (1301–2966) (680–11,042), 66 | 2010.5 (1253–2960) (680–11,042), 66 | 2038 (1301–2966) (742–8392), 67 | 2037 (1301–2960) (680–11,042), 133 |
| January–March 2017 (follow-up) | 1940 (1291–2873) (672–7439), 67 | 2031 (1266–2962) (765–11,127), 66 | 1967 (1241–2939) (672–11,127), 66 | 1984 (1318–2928) (715–8351), 67 | 1984 (1276–2928) (672–11,127), 133 |
| April–June 2017 (follow-up) | 2034 (1279–2820) (618–7666), 67 | 1968 (1299–3043) (709–10,618), 66 | 1934 (1279–2924) (708–10,618), 66 | 1979 (1327–3043) (618–8032), 67 | 1979 (1299–2924) (618–10,618), 133 |
| Total volume of RBCs wasted, median (IQR) (range), n | |||||
| Baseline | 198 (106–279) (26–1406), 66 | 154 (103–314) (31–1450), 65 | 190 (111.5–269) (32–1450), 64 | 168 (101–315) (26–1406), 67 | 184 (103–313) (26–1450), 131 |
| Follow-up | 189 (104–300) (19–1713), 65 | 155.5 (79–309) (6–1665), 66 | 187 (85–296) (6–1713), 64 | 178 (89–324) (17–1355), 67 | 180 (86–309) (6–1713), 131 |
| July–September 2015 (baseline) | 46.5 (30–75) (2–392), 64 | 37 (25–72) (7–409), 62 | 47 (31–81) (7–409), 61 | 36 (25–70) (2–392), 65 | 40.5 (28–72) (2–409), 126 |
| October–December 2015 (baseline) | 49 (24–73) (6–316), 63 | 35 (23–79) (2–427), 63 | 43 (24–73) (2–427), 61 | 46 (23–72) (6–305), 65 | 44.5 (24–73) (2–427), 126 |
| January–March 2016 (baseline) | 48.5 (24.5–75.5) (6–378), 64 | 46.5 (27.5–90) (3–346), 64 | 48.5 (27–70) (3–346), 62 | 45.5 (27–84) (5–378), 66 | 47 (27–82.5) (3–378), 128 |
| April–June 2016 (baseline) | 54 (23–79) (4–442), 65 | 43.5 (23.5–84.5) (5–268), 64 | 46 (23–75) (5–442), 63 | 49.5 (23–82) (4–331), 66 | 47 (23–80) (4–442), 129 |
| July–September 2016 (follow-up) | 50 (25.5–79) (2–458), 64 | 43 (24–89) (8–374), 63 | 43.5 (24–84) (4–458), 62 | 48 (25–87) (2–340), 65 | 44 (24–85) (2–458), 127 |
| October–December 2016 (follow-up) | 42 (25–68) (4–323), 65 | 40 (18–69) (6–438), 63 | 41.5 (20–64) (4–438), 62 | 40 (20–70) (5–295), 66 | 40.5 (20–68.5) (4–438), 128 |
| January–March 2017 (follow-up) | 45 (23–77) (2–541), 63 | 43 (20–75) (4–424), 63 | 47 (23–69) (4–541), 62 | 42 (21–83.5) (2–276), 64 | 43.5 (21–75) (2–541), 126 |
| April–June 2017 (follow-up) | 49 (28–78) (1–510), 64 | 37 (23–70.5) (2–541), 64 | 45 (25–69) (2–510), 64 | 45.5 (26–87.5) (1–541), 64 | 45 (25.5–76) (1–541), 128 |
Appendix 3 Economic analysis
For the standard versus enhanced content analyses, the ICER can be given as in Box 2.
Costs(standard content) = cost of collecting audit data and delivering standard audit report + cost of activity conducted as a result of receiving audit report + cost of blood units transfused
Benefits(standard content) = the percentage of transfusions given that were acceptable
Enhanced content audit report (intervention)Costs(enhanced content) = cost of collecting audit data and delivering enhanced audit report + cost of activity conducted as a result of receiving audit report + cost of blood units transfused
Benefits(enhanced content) = the percentage of transfusions given that were acceptable
ICER = (costs(enhanced content) – costs(standard content)) ÷ (benefits(enhanced content) – benefits(standard content))
For the standard versus enhanced follow-on support analyses, the ICER can be given as in Box 3.
Costs(standard follow-on support) = cost of collecting audit data and delivering standard audit report + cost of delivering standard follow-on support + cost of activity conducted as a result of receiving audit report + cost of blood units transfused
Benefits(standard follow-on support) = the percentage of transfusions given that were acceptable
Enhanced follow-on support (intervention)Costs(enhanced follow-on support) = cost of collecting audit data and delivering standard audit report + cost of delivering enhanced follow-on support + cost of activity conducted as a result of receiving audit report + cost of blood units transfused
Benefits(enhanced follow-on support) = the percentage of transfusions given that were acceptable
ICER = (costs(enhanced follow-on support) – costs(standard follow-on support)) ÷ (benefits(enhanced follow-on support) – benefits(standard follow-on support))
| Standard | Enhanced | Difference (i.e. additional cost of enhanced) | ||||
|---|---|---|---|---|---|---|
| Cost for all 194 sites (£) | Cost per site (£) | Cost for all 194 sites (£) | Cost per site (£) | All sites (£) | Per site (£) | |
| Content | ||||||
| Audit data collection | 110,507 | 570 | 110,507 | 570 | 0 | 0 |
| Feedback interventions | 17,069 | 88 | 60,933 | 314 | 43,864 | 226 |
| Additional NHS activity | 178,476 | 920 | 182,755 | 942 | 4278 | 22 |
| Blood transfused | 130,677,492 | 673,595 | 133,481,117 | 688,047 | 2,803,625 | 14,452 |
| Total | 130,983,544 | 675,173 | 133,835,311 | 689,873 | 2,851,768 | 14,700 |
| Follow-on support | ||||||
| Audit data collection | 110,507 | 570 | 110,507 | 570 | 0 | 0 |
| Feedback interventions | 17,069 | 88 | 30,141 | 155 | 13,073 | 67 |
| Additional NHS activity | 208,577 | 1075 | 157,169 | 810 | –51,407 | –265 |
| Blood transfused | 130,677,492 | 673,595 | 119,416,399 | 615,548 | –11,261,092 | –58,047 |
| Total | 113,064,213 | 582,805 | 119,714,217 | 617,084 | 6,650,004 | 34,278 |
| Percentage of transfusions acceptable | Volume of RBCs transfused (SD) | Mean RBCs per site (SD) | Volume of platelets transfused (SD) | Mean platelets per site (SD) | Number of SHOT events (SD) | Mean SHOT events per site (SD) | |
|---|---|---|---|---|---|---|---|
| Standard content | 74.4 | 625,753 (437,078) | 3226 (2253) | 90,122 (112,250) | 465 (579) | 1355 (1674) | 7.0 (8.6) |
| Enhanced content | 71.4 | 635,726 (456,894) | 3277 (2355) | 94,699 (130,297) | 488 (672) | 1285 (1061) | 6.6 (5.5) |
| Standard follow-on support | 73.9 | 584,232 (423,981) | 3012 (2185) | 43,714 (60,247) | 225 (311) | 1256 (914) | 6.5 (4.7) |
| Enhanced follow-on support | 72.1 | 618,725 (428,452) | 3189 (2209) | 46,438 (58,331) | 239 (301) | 1384 (1750) | 7.1 (9.0) |
Trial 2: percentage of transfusions acceptable (enhanced vs. standard content)
The PSA results suggest that the mean incremental cost of the enhanced compared with standard intervention was £23,869 per site (95% UI –£851,107 to £898,846). The mean incremental change in percentage of acceptable transfusions was –3.04% (95% UI –5.0% to –1.0%). Therefore, the enhanced intervention was more costly and less effective than the standard intervention. Figure 19 shows the simulated outputs on a CEP. The CEAC in Figure 20 suggests a downwards trend, as iterations in the south-west quadrant are found not to be cost-effective when the WTP threshold is increased.
FIGURE 19.
The CEP for enhanced vs. standard content for percentage of acceptable transfusions.
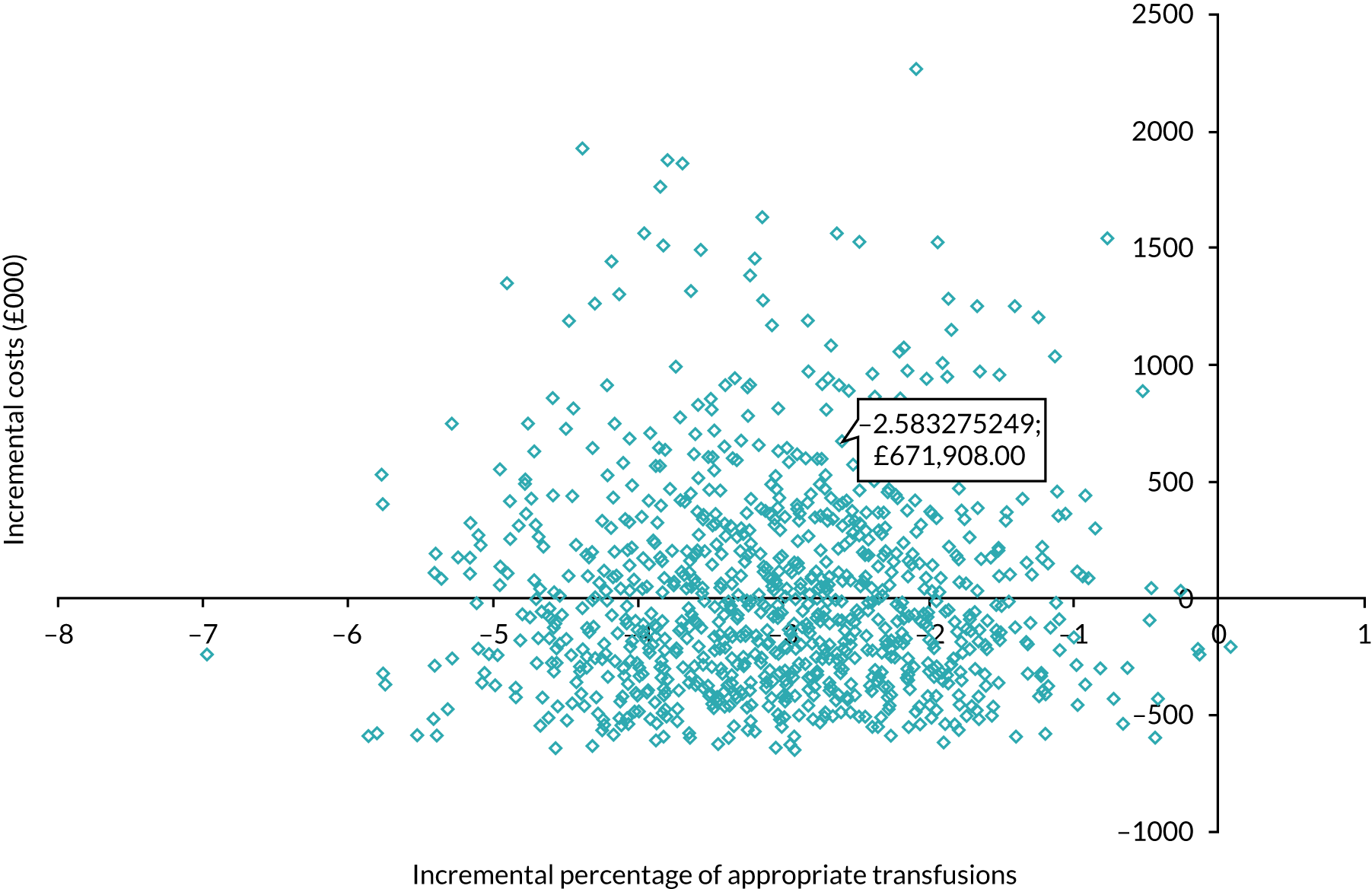
FIGURE 20.
The CEAC for enhanced vs. standard content for percentage of acceptable transfusions.
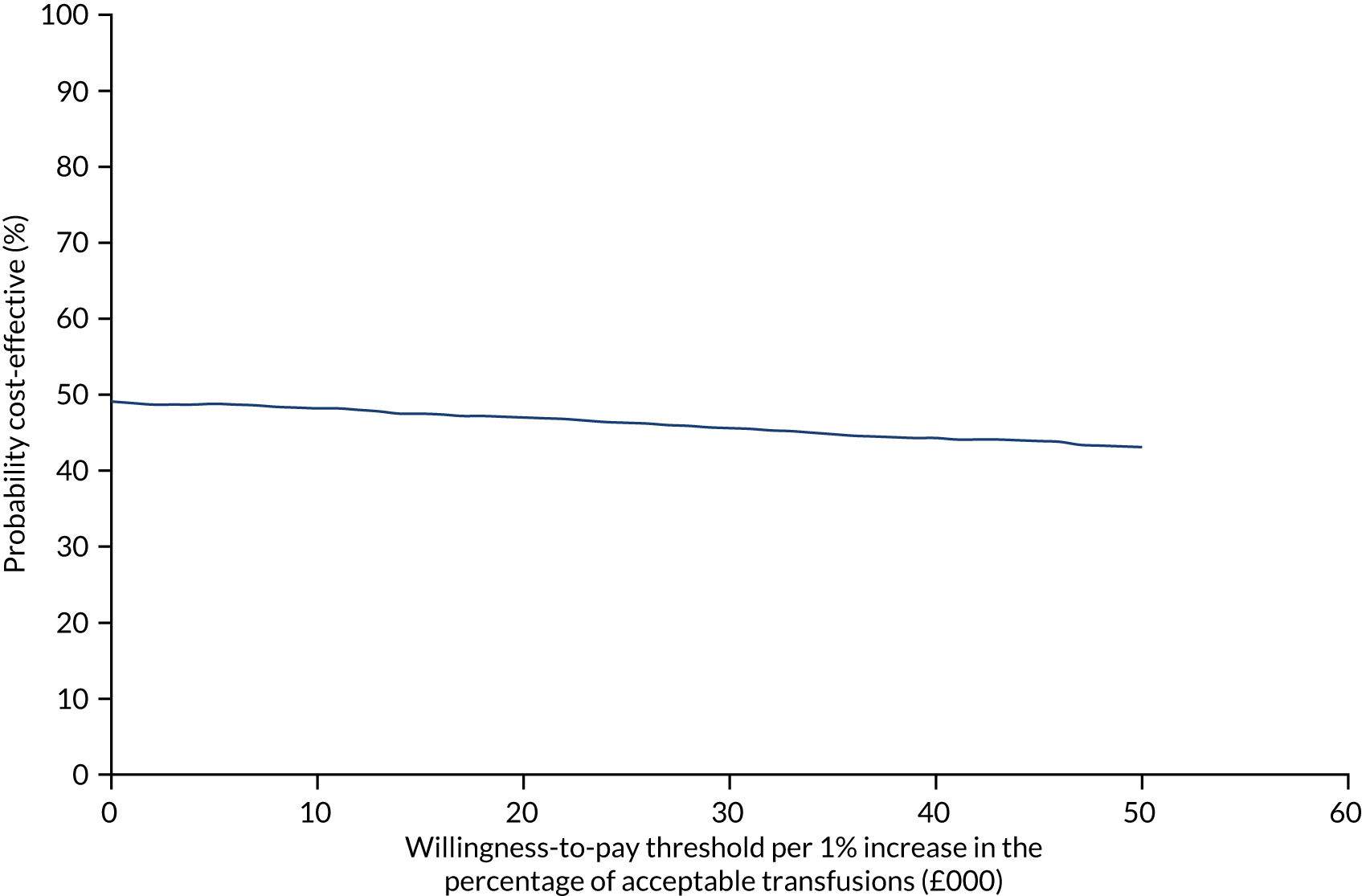
Trial 2: percentage of transfusions acceptable (enhanced vs. standard follow-on support)
The PSA results suggest that the mean incremental cost of the enhanced compared with standard intervention was £36,201 per site (95% UI –£1,045,641 to £1,118,042). The mean incremental change in percentage of acceptable transfusions was –1.8% (95% UI –3.8% to 0.2%). Therefore, the enhanced intervention was more costly and less effective than the standard intervention. Figure 21 shows the simulated outputs on a CEP. The CEAC in Figure 22 suggests a downwards trend, as iterations in the south-west quadrant are found not to be cost-effective when the WTP thresholds are increased, and comparatively few iterations in the north-east quadrant are found to be cost-effective.
FIGURE 21.
The CEP for enhanced vs. standard follow-on support for percentage of acceptable transfusions.
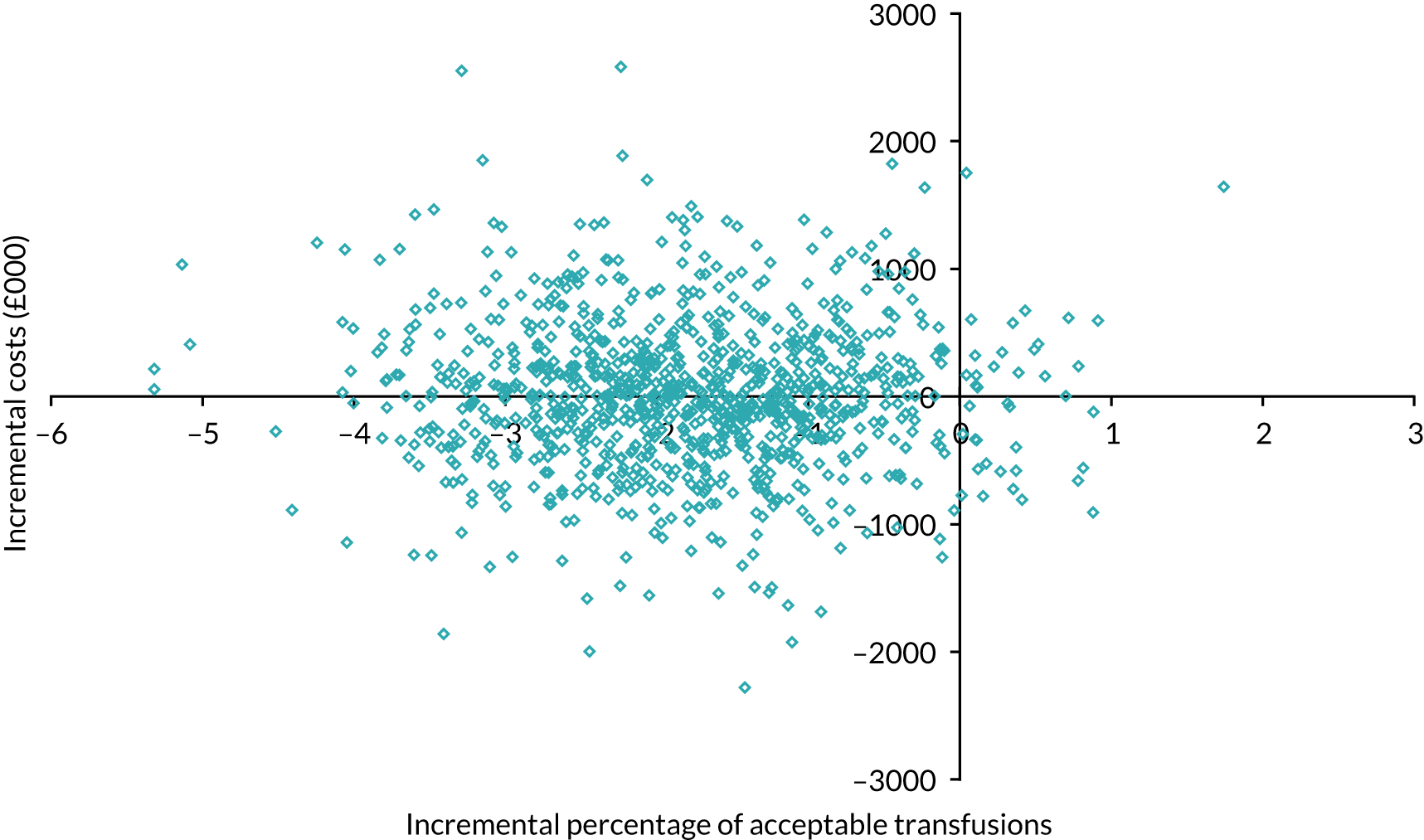
FIGURE 22.
The CEAC for enhanced vs. standard follow-on support for percentage of acceptable transfusions.
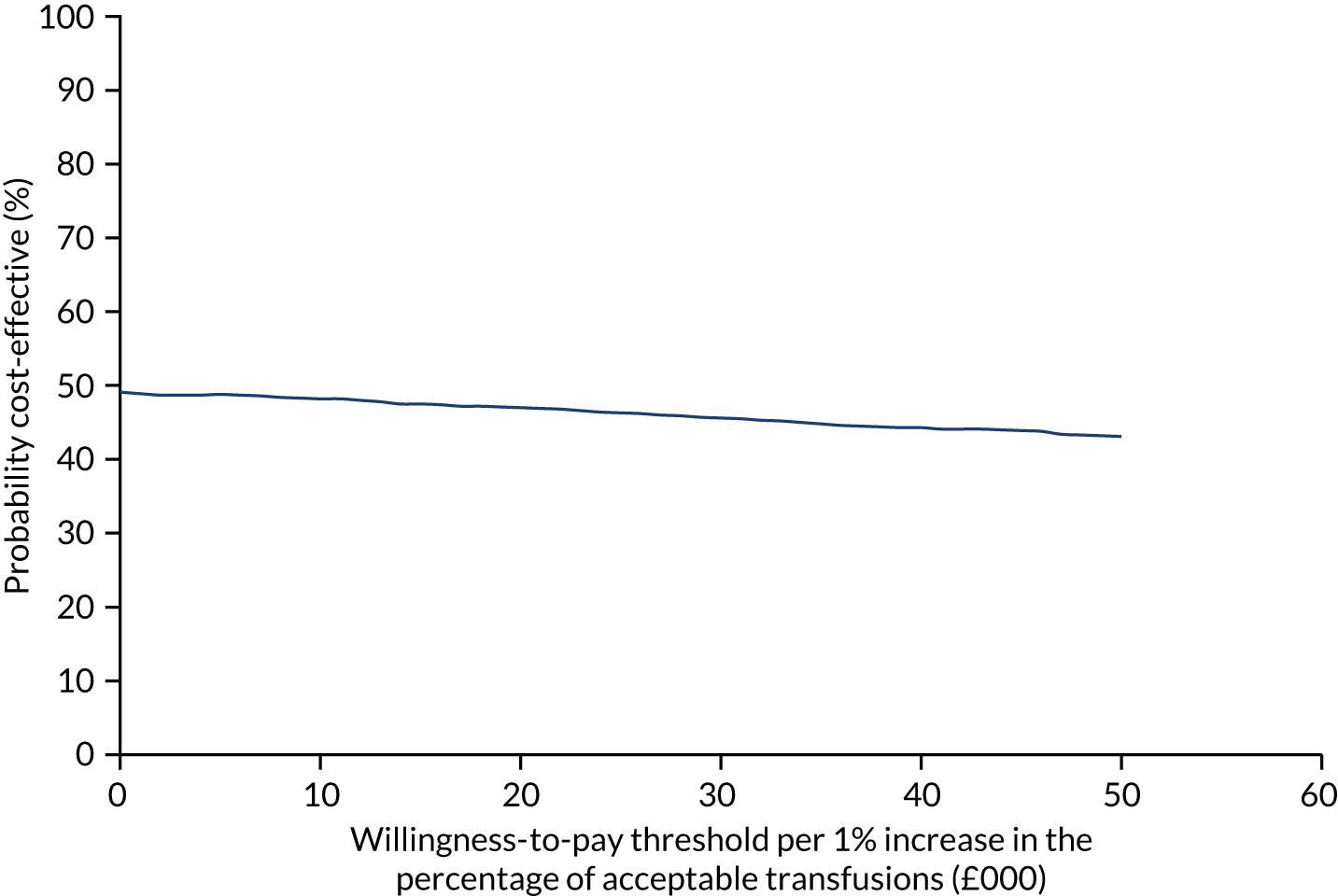
| Cost per site | Units of RBCs needed to be cost neutral | |||
|---|---|---|---|---|
| At prevailing cost of RBCs | At prevailing cost of RBCs – 20% | At prevailing cost of RBCs + 20% | ||
| Enhanced vs. standard content | ||||
| Incremental mean | £248 | 1.4 | 1.8 | 1.2 |
| 95% UI upper bound | £698 | 4.0 | 5.0 | 3.3 |
| 95% UI lower bound | –£202 | –1.1 | –1.4 | –1.0 |
| Enhanced vs. standard follow-on support | ||||
| Incremental mean | –£198 | –1.1 | –1.4 | –0.9 |
| 95% UI upper bound | –£161 | –0.9 | –1.1 | –0.8 |
| 95% UI lower bound | –£234 | –1.3 | –1.7 | –1.1 |
Appendix 4 Process evaluation interview participant demographics
| Participant demographics | Trial 1 | Trial 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enhanced content and support | Enhanced content | Enhanced support | Routine practice | All arms | Enhanced content and support | Enhanced content | Enhanced support | Routine practice | All arms | |
| Number of participants | 11 | 5 | 7 | 12 | 35 | 5 | 5 | 6 | 4 | 20 |
| Number of clusters | 6 | 4 | 5 | 6 | 21 | 3 | 4 | 4 | 3 | 14 |
| Years working at site, n (%) | ||||||||||
| 0–4 | 3 (27.3) | 0 (00.0) | 1 (14.3) | 3 (25.0) | 7 (20.0) | 2 (40.0) | 2 (40.0) | 3 (50.0) | 1 (25.0) | 8 (40.0) |
| 5–14 | 5 (45.4) | 3 (60.0) | 5 (71.4) | 8 (66.7) | 21 (60.0) | 2 (40.0) | 2 (40.0) | 2 (33.3) | 2 (50.0) | 8 (40.0) |
| ≥ 15 | 3 (27.3) | 2 (40.0) | 1 (14.3) | 1 (8.3) | 7 (20.0) | 1 (20.0) | 1 (20.0) | 1 (16.7) | 1 (25.0) | 4 (20.0) |
| Makes transfusion decisions?, n (%) | ||||||||||
| Yes | 5 (53.8) | 2 (40.0) | 2 (28.6) | 6 (50.0) | 15 (42.9) | 3 (60.0) | 1 (20.0) | 1 (16.7) | 4 (100.0) | 9 (45.0) |
| No | 6 (46.2) | 3 (60.0) | 5 (71.4) | 6 (50.0) | 82 (57.1) | 2 (40.0) | 4 (60.0) | 5 (83.3) | 0 (00.0) | 11 (55.0) |
| Professional role, n (%) | ||||||||||
| Transfusion practitioner | 4 (36.4) | 4 (80.0) | 5 (71.4) | 6 (50.0) | 19 (54.3) | 2 (60.0) | 2 (40.0) | 4 (66.7) | 2 (50.0) | 10 (50.0) |
| Othera | 7 (63.6) | 1 (20.0) | 2 (28.6) | 6 (50.0) | 16 (45.7) | 3 (40.0) | 3 (60.0) | 2 (33.3) | 2 (50.0) | 10 (50.0) |
Appendix 5 Workstream 4: the development of general implementation recommendations and tools for relevant audit and feedback programmes in the wider NHS
Our original programme proposal set out plans for a structured stakeholder consultation, which was to lead to the following outputs:
-
evidence-based materials and resources to support intervention adaptation and scaling up for other national audits
-
clear specifications of the intervention components, the professionals targeted, the fidelity of the interventions and the mechanisms of change made available through publications and presentations
-
the establishment of a bank of resources, including the tools used for the interventions, with practical steps to operationalise and a methodology for assuring the validity of audit data.
We proposed inviting clinical and management leads involved in the transfusion audits and other national audit programmes, as well as members of the PPI panel created for this programme, to a series of four ‘roundtable’ meetings.
As the programme progressed, we found that developing relationships with and offering further advice to a number of national audit programmes, as well as working as much as possible within existing networks, offered more fruitful approaches to engagement and dissemination than hosting further ‘roundtable’ events. We therefore changed our approach (highlighted in our 2017 report to NIHR) as follows:
-
engagement with the HQIP and allied national clinical audit programmes
-
conducting and sharing audits of feedback methods used by national audit programmes (‘audit of audits’)
-
international collaborative meetings for audit and feedback providers, commissioners and researchers (leading to the ‘A&F MetaLab’)
-
holding a national dissemination event in partnership with HQIP.
Although we recognise that both feedback interventions (enhanced content and enhanced follow-on) were ineffective when evaluated in the trials, we have made relevant intervention materials available (see Report Supplementary Material 1–27) because they nevertheless cover important points for audit programmes to consider when designing and delivering feedback.
Engagement with Healthcare Quality Improvement Partnership and national clinical audits
We decided to channel the majority of our engagement activities with national audit programmes through HQIP, given that it is responsible for commissioning most national audits in the UK, holds regular update events and distributes guidance on audit methods. We (RF) joined and attended the HQIP Methodology Advisory Group from 2017 onwards. This allowed us to gain an understanding of the key methodological issues facing national audits (e.g. ensuring data validity, promoting local action following feedback). We shared emerging lessons from AFFINITIE and updates of evidence on effective feedback at HQIP seminars and with individual audit programmes, including:
-
Trauma Audit and Research Network, selected executive members, November 2016
-
National Clinical Audit and Patient Outcomes Programme (NCAPOP) spring seminar, May 2017
-
National Diabetes Audit executive, September 2017
-
National Airways Audit executive, September 2017
-
Paediatric Intensive Care Audit Network (PICANet), November 2017
-
National Neonatal Audit Programme (NNAP) & Neonatal Data Analysis Unit (NDAU) Annual Collaborators’ Meeting, April 2018
-
HQIP Methodology Advisory Group, November 2018.
Audit of audits
In November 2015, we identified a baseline sample of national audit reports for 23 programmes listed on the HQIP websites. We applied a set of evidence-based and good practice criteria to these reports. We verified our assessments, where possible, with national audit leads and project managers. HQIP published Reporting for Impact Guidance, to enhance the impact of national audits in March 2016. 38 We repeated our assessment in January 2017 by applying the criteria to a follow-up sample of 20 re-audit reports (out of the original 23 national audit programmes).
We identified a range of improvements over time in the content of audit reports, for example in the identification of key audit standards, findings and recommendations, the definition of target groups for dissemination, the use of comparators and achievable benchmarks, and the presentation and specification of action plans (Table 30). We attribute these to HQIP rather than to our AFFINITIE activities. We also identified areas for improvement (e.g. reducing time intervals between data collection and feedback). We reported our findings directly to HQIP and shared them with international collaborator meetings. With further refinements, a criterion-based ‘audit of audits’ offers an efficient means of monitoring the quality of national audit reports.
| Domain | Criterion | Audit programmes meeting criterion | |||
|---|---|---|---|---|---|
| Baseline | Follow-up | ||||
| n | Proportion (%) | n | Proportion (%) | ||
| Audit components | Data based on recent performance (< 6 months) | 2 | 9 | 1 | 5 |
| Audit cycles repeated or intended to be repeated | 21 | 91 | 19 | 95 | |
| Data included about the individual’s or team’s own behaviour(s) | 18 | 78 | 16 | 80 | |
| Importance of audit topic as related to patient care clearly stated | 22 | 96 | 20 | 100 | |
| Feedback components | Authorship of the feedback report identified as a trusted source (e.g. recognised professional body) | 23 | 100 | 20 | 100 |
| A specific dissemination list provided for the feedback report | 4 | 17 | 18 | 90 | |
| Multimodal presentation including either text and talking or text and graphical materials | 23 | 100 | 19 | 95 | |
| National data displayed in graphical form | 21 | 91 | 18 | 90 | |
| Regional data displayed in graphical form | 13 | 57 | 10 | 50 | |
| A short or summarised version of the feedback report is available online | 1 | 4 | 5 | 25 | |
| Key audit standards present | 18 | 78 | 18 | 90 | |
| Key audit standards easily identified in the document (e.g. highlighted text or box) | 14 | 61 | 18 | 90 | |
| Key audit findings present | 23 | 100 | 20 | 100 | |
| Key audit findings easily identified in the document (e.g. highlighted text or box) | 18 | 78 | 20 | 100 | |
| Audit recommendations present | 18 | 78 | 19 | 95 | |
| Audit recommendations easily identified in the document (e.g. highlighted text or box) | 15 | 65 | 19 | 95 | |
| Enhanced feedback | Recommendations clearly linked to audit standards | 6 | 26 | 16 | 80 |
| Action plans phrased in a behaviourally specific manner (who, what, when, where) | 9 | 39 | 19 | 95 | |
| Actions plans easily identified in the document (e.g. highlighted text or box) | 9 | 39 | 17 | 85 | |
| Positive feedback highlighted when a standard has been achieved or when there has been significant improvement since a previous audit | 10 | 43 | 9 | 45 | |
| Feedback includes multiple comparators for national performance | Audit standards | 12 | 52 | 18 | 90 |
| Past performance | 18 | 78 | 17 | 85 | |
| Achievable benchmark (e.g. top 10%) | 2 | 9 | 8 | 40 | |
| Regional comparators | 11 | 48 | 15 | 75 | |
| Feedback includes multiple comparators for regional performance | Audit standards | 4 | 17 | 14 | 70 |
| Past performance | 5 | 22 | 9 | 45 | |
| Achievable benchmarks (e.g. top 10%) | 0 | 0 | 9 | 45 | |
| Regional comparators | 18 | 78 | 15 | 75 | |
| National average | 12 | 52 | 14 | 70 | |
The Audit and Feedback MetaLab
We are co-founders of this international collaboration, led by our co-investigator Jeremy Grimshaw. The Audit and Feedback MetaLab (www.ohri.ca/auditfeedback/) is an international research and health-care community that aims to synthesise and share evidence on A&F, engage with health system partners and provide a trusted source of evidence and recommendations, and develop research capacity and practical expertise in A&F. We have held annual meetings to bring together researchers and audit leaders in Europe and North America since 2014. The 2017 Leeds meeting included presentations from HQIP and national audit programmes, evidence updates, and discussions about challenges faced by national audits.
Joint seminar with Healthcare Quality Improvement Partnership: what can national clinical audits learn from the AFFINITIE research programme about improving impact?
We asked HQIP to host the main dissemination seminar for AFFINITIE because we recognised that this would lend credibility and extend our reach to national audits. The seminar aimed to identify lessons for national clinical audit programmes based on AFFINITIE findings and experience.
We invited national audit leads, members of the public and researchers. In total, 99 delegates registered for the seminar, which took place in London in June 2019. We presented the trial and process evaluation findings, contextualised our work within the wider evidence base, and sought feedback on materials and toolkits produced as part of AFFINITIE.
Key lessons for national clinical audit programmes
We asked participants to suggest key lessons for national clinical audit programmes based on the study findings. Responses often echoed findings from the intervention development work and the process evaluation; for example:
Clear line of communication to different groups is essential.
Need to line up top down to the bottom up, bodies responsible for quality improvement implementation.
Audits need to support audit cycle.
Timely and continuous data collection.
Reports with different data depending on what’s needed for role (e.g. Trust board, Managers, Clinical Team).
Is there the opportunity to embed behaviour change techniques?
Tailoring targets – what is the key behaviour change we’re after?
Ensure your audit measures are credible, i.e. based on evidence, NICE guidance expert consensus.
Don’t expect teams to be proactive about finding reports and toolkits.
The credibility of the audit (as locally perceived) can influence how useful/effective the feedback is.
Content and format of intervention materials
We provided participants with one of three materials from AFFNITIE: an example of a short feedback report, a copy of the brief enhancement guidance or selected screenshots from the online support toolkit. We asked for suggested improvements to the content or format, most of which related to the need to tailor intervention materials to the needs of local users and provide more examples:
Dense and intimidating.
Too much text . . . Dry academic style. Assumes knowledge about quality improvement, e.g. Fishbone [diagram].
More detail in the recommendations.
Sample action plan.
Overall evaluation and future intentions
Out of 36 respondents, 31 found the presentation of the study results extremely or mainly useful and 29 found the overall event extremely or mainly useful.
We asked participants if there was any aspect of their work that may review and change. A number of responses suggested intentions to review and change audit methods or even take part in further research:
Need to target more and reduce contact reports. Need to think about joining up national voice to promote key messages.
Consider how to make quality improvement messages clear and smart.
We will review the whole audit design process and focus on the way we engage with NHS Trusts.
Considering alternative comparators.
We will review our plans to mandate action plans following publication of audit results.
How to take forward an implementation lab.
Summary
We managed a sustained engagement campaign with evidence of good reach to interested parties, especially the commissioner (HQIP) and providers of national audit programmes. We have actively shared emerging evidence on A&F, including from work beyond AFFINITIE, and indicated scope for improvements in the design and conduct of national audits. Feedback from seminar participants, taken in context with the wider AFFINITIE programme results, suggests that there is a need for greater involvement of end-users in the final design of feedback interventions.
List of abbreviations
- AACTT
- Actor, Action, Context, Target, Timeframe
- A&F
- audit and feedback
- AFFINITIE
- Development & Evaluation of Audit and Feedback INterventions to Increase evidence-based Transfusion practice
- BCT
- behaviour change technique
- BSMS
- Blood Stocks Management Scheme database
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- DSA
- deterministic sensitivity analysis
- HQIP
- Healthcare Quality Improvement Partnership
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NCA
- National Comparative Audit
- NCABT
- National Comparative Audit of Blood Transfusion
- NHSBT
- NHS Blood and Transplant
- NICE
- National Institute for Health and Care Excellence
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health Research
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- SHOT
- Serious Hazards of Transfusion database
- TDF
- theoretical domains framework
- TIDieR
- Template for Intervention Description and Replication
- UI
- uncertainty interval
- WS
- workstream
- WTP
- willingness to pay
Notes
-
WS1a coding framework for specifying the content and characteristics of existing audit and feedback reports from the National Comparative Audit of Blood Transfusions
-
Topic guide for WS1b semistructured interviews to explore how hospitals respond to feedback received from the National Comparative Audit of Blood Transfusion and implement change in light of feedback
-
Structured observation sheet for use during observation of meetings where blood transfusion audit and feedback is discussed
-
Behaviour Change Techniques (BCTs) identified at least once across all included feedback documents, presented in ranked order from highest to lowest proportion of feedback documents identified in
-
Behavioural specification of the audit standards, feedback and recommendations identified in existing feedback reports, presented according to the AACTT principle of behavioural specificity (Presseau et al. , in press)
-
Presence of evidence-based feedback characteristics and components across feedback documents within each of the three audits
-
2015 Audit of Patient Blood Management in adults undergoing elective, scheduled surgery: Key Findings Report
-
2015 Audit of Patient Blood Management in adults undergoing elective, scheduled surgery: Full Findings Report
-
2015 Audit of Patient Blood Management in adults undergoing elective, scheduled surgery: Supplementary Information Report
-
AFFINITIE full guidance for enhancing feedback reports following an audit: July 2015
-
AFFINITIE brief guidance for enhancing feedback reports following an audit: July 2015
-
Dissemination pathways representing who currently receives feedback from a national comparative audit of blood transfusion
-
WS1B Themes from interviews with health-care professionals from four case study hospitals regarding barriers and enables to responding to feedback and implementing change in light of feedback
-
Behaviour Change Techniques included in the Enhanced Follow-on intervention Toolkit and Telephone Support
-
Screenshots of final version of Intervention 2 – Enhanced Follow On, web-based Toolkit
-
Intervention 1 – Enhanced Follow-on: Summary results from piloting and feasibility interviews
-
Intervention 2 –Enhanced Follow-on: Summary results from piloting and feasibility interviews
-
Template for Intervention Description and Replication Checklist
-
Secondary Outcomes Results for both Trials (cost-effectiveness and sensitivity analysis)
-
Presence of six proposed enhancements to design and content of feedback reports in Enhanced Content intervention reports in Trial 1 and 2
-
Fidelity of delivery of manual-specific behaviour change techniques for Enhanced Follow-On initial telephone support
-
Descriptive cluster-level statistics (median, range) across both arms for engagement with pages/tools in the toolkit in Trial 1 & 2
-
Themes related to fidelity of receipt across Enhanced and Standard feedback content interventions in Trial 1
-
Themes related to contextual influences across all interventions/trial arms in Trial 1
-
Themes related to fidelity of receipt and enactment from process evaluation interviews in Trial 2
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/REHP1241).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
